Mucosal Atomization Devices Market Report
Published Date: 31 January 2026 | Report Code: mucosal-atomization-devices
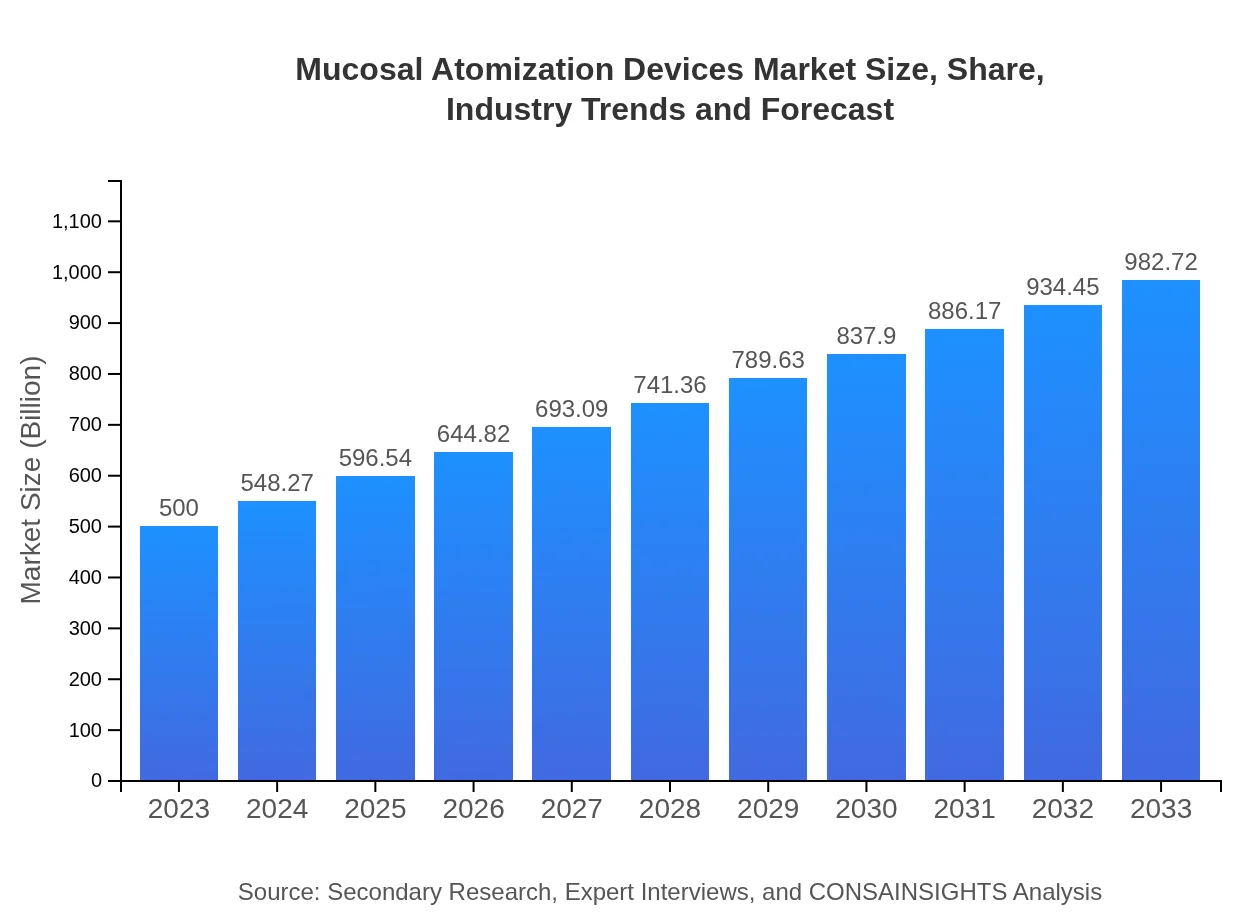
Mucosal Atomization Devices Market Size, Share, Industry Trends and Forecast to 2033
This report provides an in-depth analysis of the Mucosal Atomization Devices market, covering essential insights, segmentation, and growth forecasts for the period 2023 to 2033.
| Metric | Value |
|---|---|
| Study Period | 2023 - 2033 |
| 2023 Market Size | $500.00 Million |
| CAGR (2023-2033) | 6.8% |
| 2033 Market Size | $982.72 Million |
| Top Companies | Teleflex Inc., BD (Becton, Dickinson and Company), Medtronic plc, Owen Mumford Ltd. |
| Last Modified Date | 31 January 2026 |
Mucosal Atomization Devices Market Overview
Customize Mucosal Atomization Devices Market Report market research report
- ✔ Get in-depth analysis of Mucosal Atomization Devices market size, growth, and forecasts.
- ✔ Understand Mucosal Atomization Devices's regional dynamics and industry-specific trends.
- ✔ Identify potential applications, end-user demand, and growth segments in Mucosal Atomization Devices
What is the Market Size & CAGR of Mucosal Atomization Devices market in 2023?
Mucosal Atomization Devices Industry Analysis
Mucosal Atomization Devices Market Segmentation and Scope
Tell us your focus area and get a customized research report.
Mucosal Atomization Devices Market Analysis Report by Region
Europe Mucosal Atomization Devices Market Report:
Europe's market is set to grow from $146.30 million in 2023 to $287.54 million by 2033, supported by government initiatives to improve healthcare infrastructure and the need for advanced treatment options.Asia Pacific Mucosal Atomization Devices Market Report:
The Asia Pacific region demonstrates robust growth potential, with a projected market size of $187.40 million by 2033, up from $95.35 million in 2023. The increasing prevalence of diseases and expanded healthcare access in emerging economies are key drivers.North America Mucosal Atomization Devices Market Report:
North America maintains the highest market value, anticipated to soar from $178.50 million in 2023 to $350.83 million in 2033. The rise in chronic diseases and a substantial focus on technological advancements significantly contribute to this growth.South America Mucosal Atomization Devices Market Report:
In South America, the market is expected to grow from $10.35 million in 2023 to $20.34 million by 2033 as healthcare modernization efforts continue to improve treatment accessibility.Middle East & Africa Mucosal Atomization Devices Market Report:
The Middle East and Africa region is projected to increase from $69.50 million in 2023 to $136.60 million by 2033, driven by healthcare reforms and rising demand for efficient medical devices.Tell us your focus area and get a customized research report.
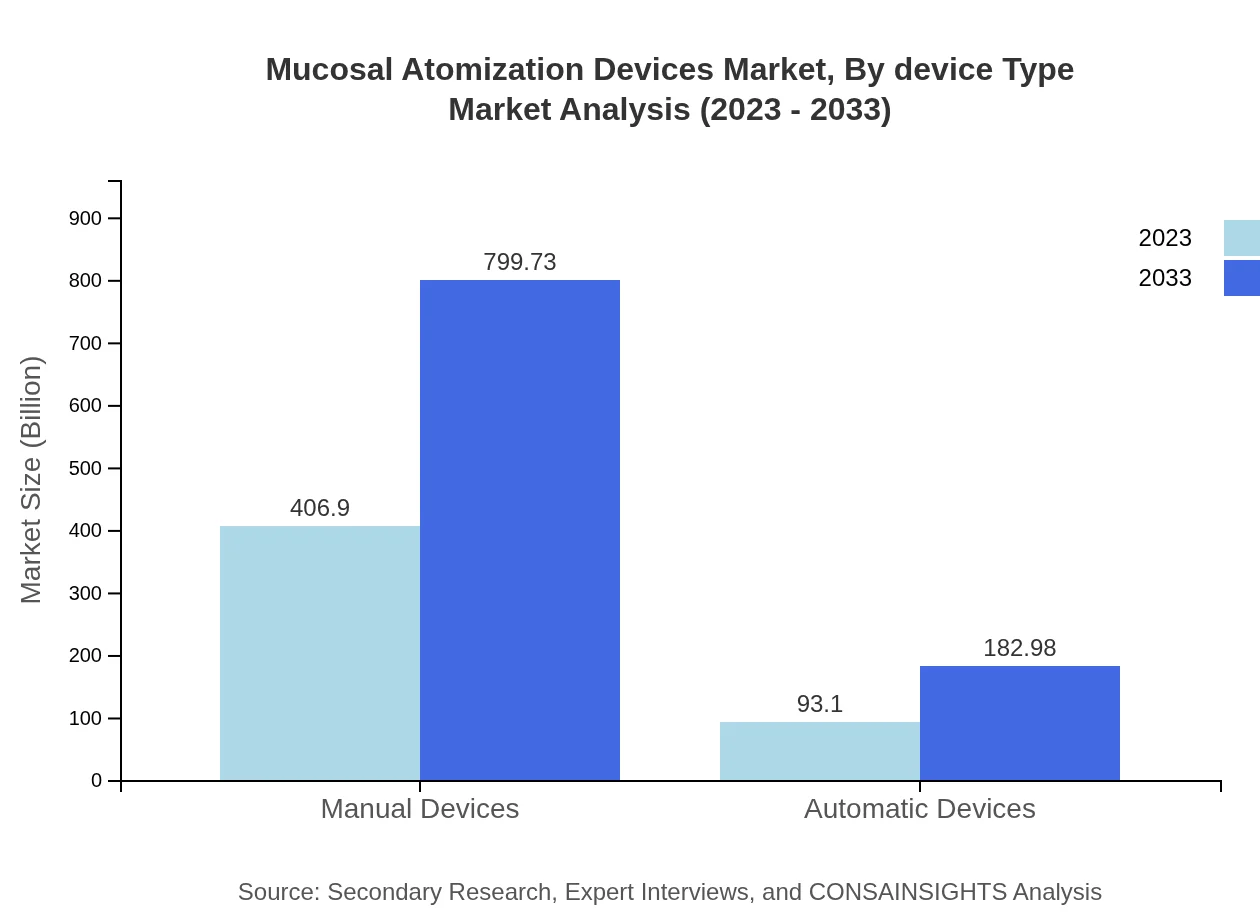
Mucosal Atomization Devices Market Analysis By Device Type
The market is significantly divided into manual and automatic devices. Manual devices are expected to dominate, with a market size of $406.90 million in 2023, anticipated to reach $799.73 million by 2033. Automatic devices, while lower in size at $93.10 million in 2023, are forecasted to grow to $182.98 million by 2033 as advancements in technology make these more accessible and desirable.
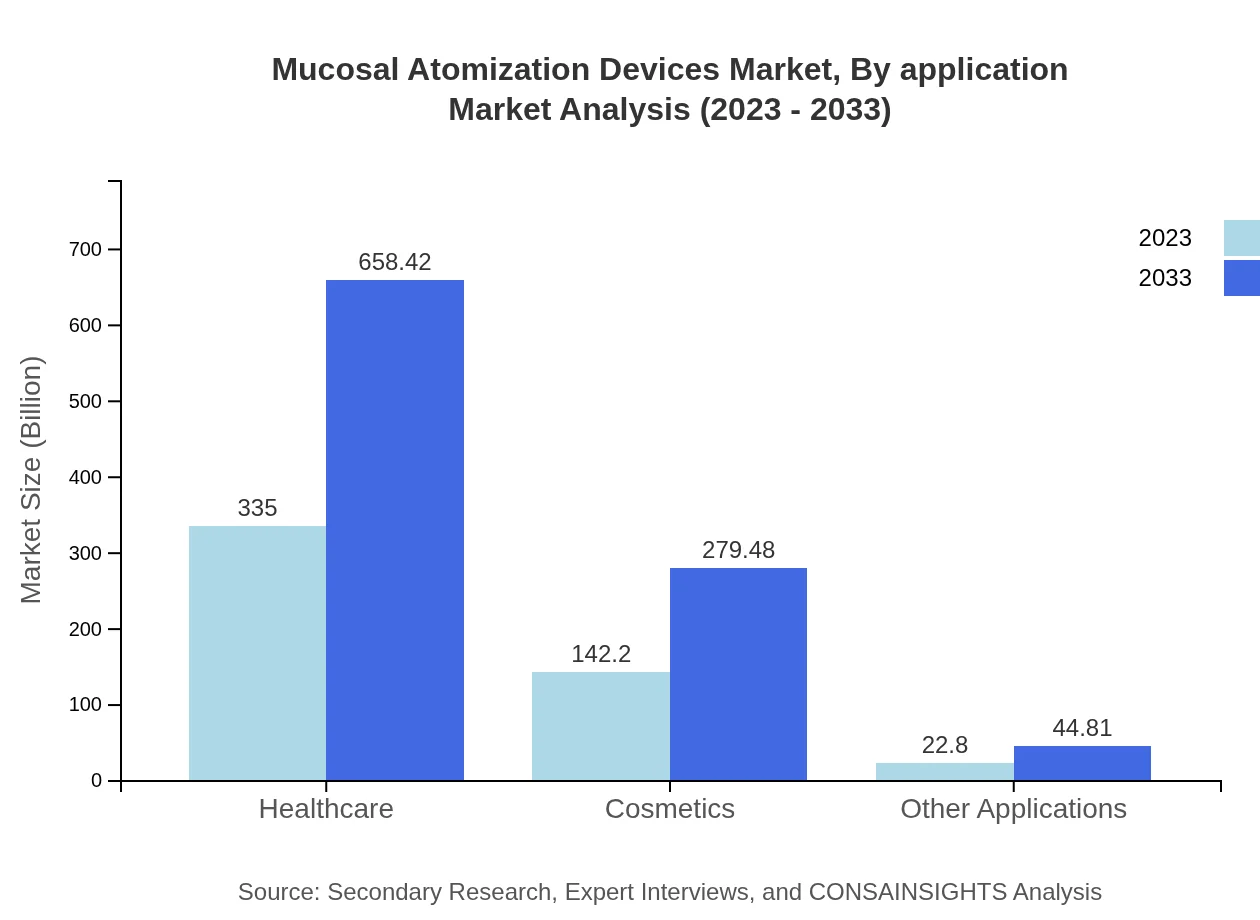
Mucosal Atomization Devices Market Analysis By Application
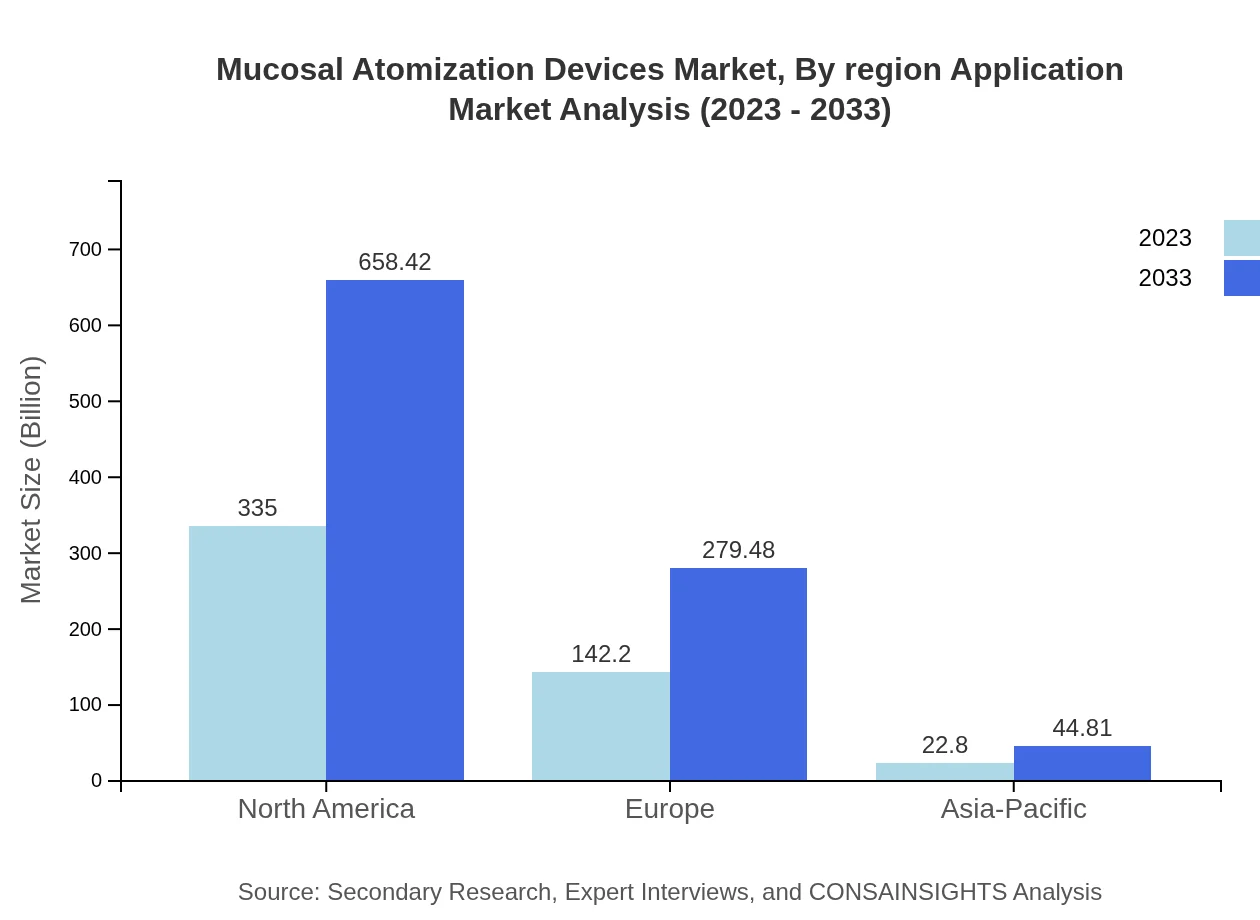
Applications are categorized into hospitals, home care, pharmaceuticals, cosmetics, and other applications. Hospitals lead with a size of $335 million in 2023, projected to grow to $658.42 million by 2033. Home care follows with a market size of $142.20 million in 2023, expected to reach $279.48 million by 2033, reflecting the increasing shift towards at-home healthcare solutions.
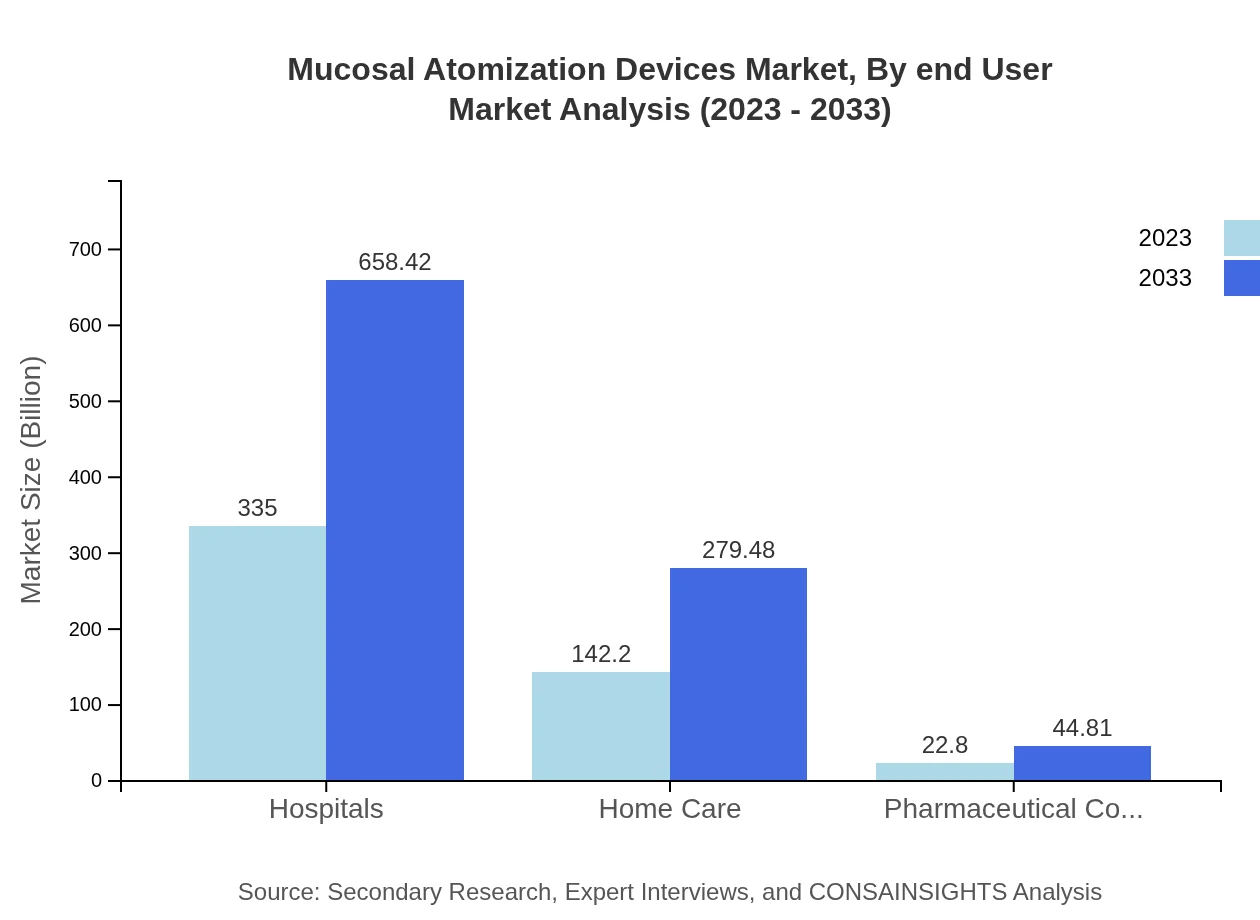
Mucosal Atomization Devices Market Analysis By End User
End-users prominently include healthcare providers, pharmaceutical companies, and consumer markets. Hospitals constitute 67% of the market share in 2023, remaining stable throughout the forecast period. The home care sector's growth reflects evolving patient care preferences and advancements in ease-of-use devices.
Mucosal Atomization Devices Market Analysis By Region Application
Regional dynamics highlight the different growth trajectories. North America holds a substantial market share, followed by Europe and Asia Pacific, leveraging innovations and larger healthcare budgets. Meanwhile, South America and the Middle East & Africa are emerging markets showing promising growth rates due to increased healthcare investments.
Mucosal Atomization Devices Market Trends and Future Forecast
Tell us your focus area and get a customized research report.
Global Market Leaders and Top Companies in Mucosal Atomization Devices Industry
Teleflex Inc.:
Teleflex is a leading provider of medical technologies, specializing in innovative and minimally invasive medical devices, including mucosal atomization solutions.BD (Becton, Dickinson and Company):
BD is a global medical technology company committed to improving safety and enhancing the quality of care. They offer a range of mucosal atomization devices for effective drug delivery.Medtronic plc:
Medtronic is a premier medical technology company known for its diverse range of advanced healthcare products including mucosal atomization devices tailored for unique patient needs.Owen Mumford Ltd.:
Owen Mumford specializes in the design and manufacturing of medical devices, with a focus on user-centered designs to enhance patient experience in drug delivery.We're grateful to work with incredible clients.









FAQs
What is the market size of mucosal Atomization Devices?
The mucosal atomization devices market is projected to grow from a size of approximately $500 million in 2023 to significant levels by 2033, with a CAGR of 6.8%, indicating a robust increase in demand over the ten-year period.
What are the key market players or companies in this mucosal Atomization Devices industry?
Key players in the mucosal atomization devices market include established medical device manufacturers as well as innovative startups that focus on developing advanced nebulization technologies aimed at enhancing drug delivery through mucosal routes.
What are the primary factors driving the growth in the mucosal Atomization Devices industry?
The growth of the mucosal atomization devices market is primarily driven by the increasing demand for pain-free drug delivery, advancements in medical technology, and the rising prevalence of respiratory and chronic diseases globally.
Which region is the fastest Growing in the mucosal Atomization Devices?
In the mucosal atomization devices market, North America is currently the fastest-growing region, with market size expected to expand from $178.50 million in 2023 to $350.83 million by 2033, reflecting a strong CAGR amid rising healthcare investments.
Does ConsaInsights provide customized market report data for the mucosal Atomization Devices industry?
Yes, ConsaInsights offers customized market report data tailored to specific needs within the mucosal atomization devices industry, enabling clients to access detailed analytics, market trends, and projections according to their unique strategic goals.
What deliverables can I expect from this mucosal Atomization Devices market research project?
Deliverables from this market research project include comprehensive reports, detailed market analysis, strategic insights, competitor benchmarking, and forecasts segmented by region, application, and device type for mucosal atomization devices.
What are the market trends of mucosal Atomization Devices?
Current market trends for mucosal atomization devices include an increasing shift towards home healthcare settings, rising popularity of automatic devices, and innovations focusing on enhancing patient compliance and the efficacy of drug delivery systems.