Multiple Myeloma Therapeutics Market Report
Published Date: 31 January 2026 | Report Code: multiple-myeloma-therapeutics
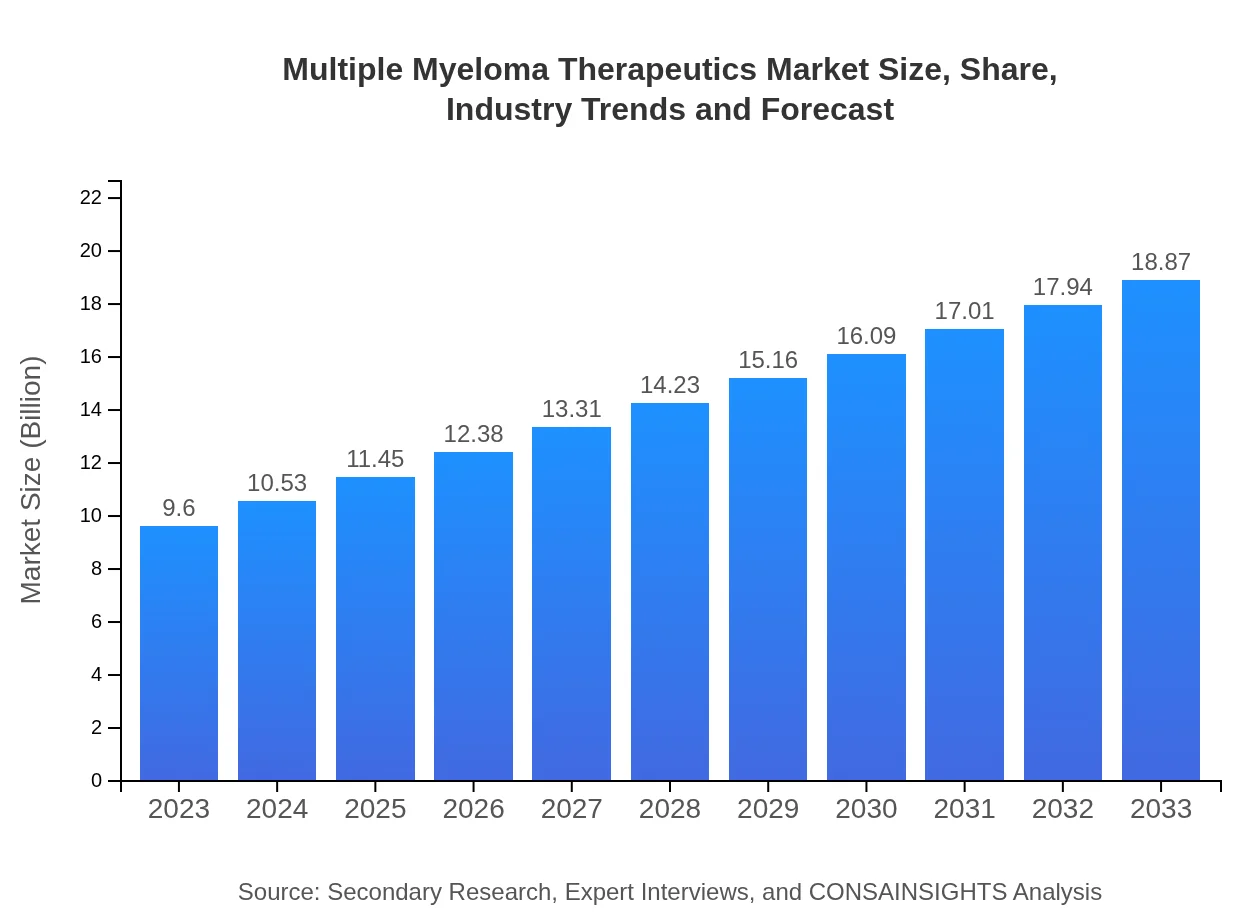
Multiple Myeloma Therapeutics Market Size, Share, Industry Trends and Forecast to 2033
This report provides a comprehensive analysis of the Multiple Myeloma Therapeutics market from 2023 to 2033, detailing insights into market size, growth trends, industry analysis, and regional performance.
| Metric | Value |
|---|---|
| Study Period | 2023 - 2033 |
| 2023 Market Size | $9.60 Billion |
| CAGR (2023-2033) | 6.8% |
| 2033 Market Size | $18.87 Billion |
| Top Companies | Celgene Corporation, Johnson & Johnson, Amgen Inc., AbbVie Inc., Bristol-Myers Squibb |
| Last Modified Date | 31 January 2026 |
Multiple Myeloma Therapeutics Market Overview
Customize Multiple Myeloma Therapeutics Market Report market research report
- ✔ Get in-depth analysis of Multiple Myeloma Therapeutics market size, growth, and forecasts.
- ✔ Understand Multiple Myeloma Therapeutics's regional dynamics and industry-specific trends.
- ✔ Identify potential applications, end-user demand, and growth segments in Multiple Myeloma Therapeutics
What is the Market Size & CAGR of Multiple Myeloma Therapeutics market in 2033?
Multiple Myeloma Therapeutics Industry Analysis
Multiple Myeloma Therapeutics Market Segmentation and Scope
Tell us your focus area and get a customized research report.
Multiple Myeloma Therapeutics Market Analysis Report by Region
Europe Multiple Myeloma Therapeutics Market Report:
In Europe, the market is anticipated to grow from $2.31 billion in 2023 to $4.54 billion by 2033. The growth can be attributed to supportive regulatory frameworks, increasing healthcare spending, and rising incidence rates of Multiple Myeloma.Asia Pacific Multiple Myeloma Therapeutics Market Report:
The Asia-Pacific region is expected to witness steady growth from $1.85 billion in 2023 to $3.63 billion by 2033. This growth is attributed to rising healthcare expenditures and improved access to treatments, alongside an increasing awareness of Multiple Myeloma.North America Multiple Myeloma Therapeutics Market Report:
North America dominates the Multiple Myeloma therapeutics market, with a projected increase from $3.59 billion in 2023 to $7.05 billion by 2033, driven by robust R&D activities, significant investments in novel therapies, and a well-established healthcare system.South America Multiple Myeloma Therapeutics Market Report:
The South American market is projected to grow from $0.71 billion in 2023 to $1.40 billion by 2033. The key drivers include increased investment in healthcare infrastructure and a growing patient base seeking advanced treatment options.Middle East & Africa Multiple Myeloma Therapeutics Market Report:
The Middle East and Africa region is expected to see growth from $1.15 billion in 2023 to $2.25 billion by 2033. This growth is driven by improving healthcare access and increasing awareness about Multiple Myeloma and its treatment.Tell us your focus area and get a customized research report.
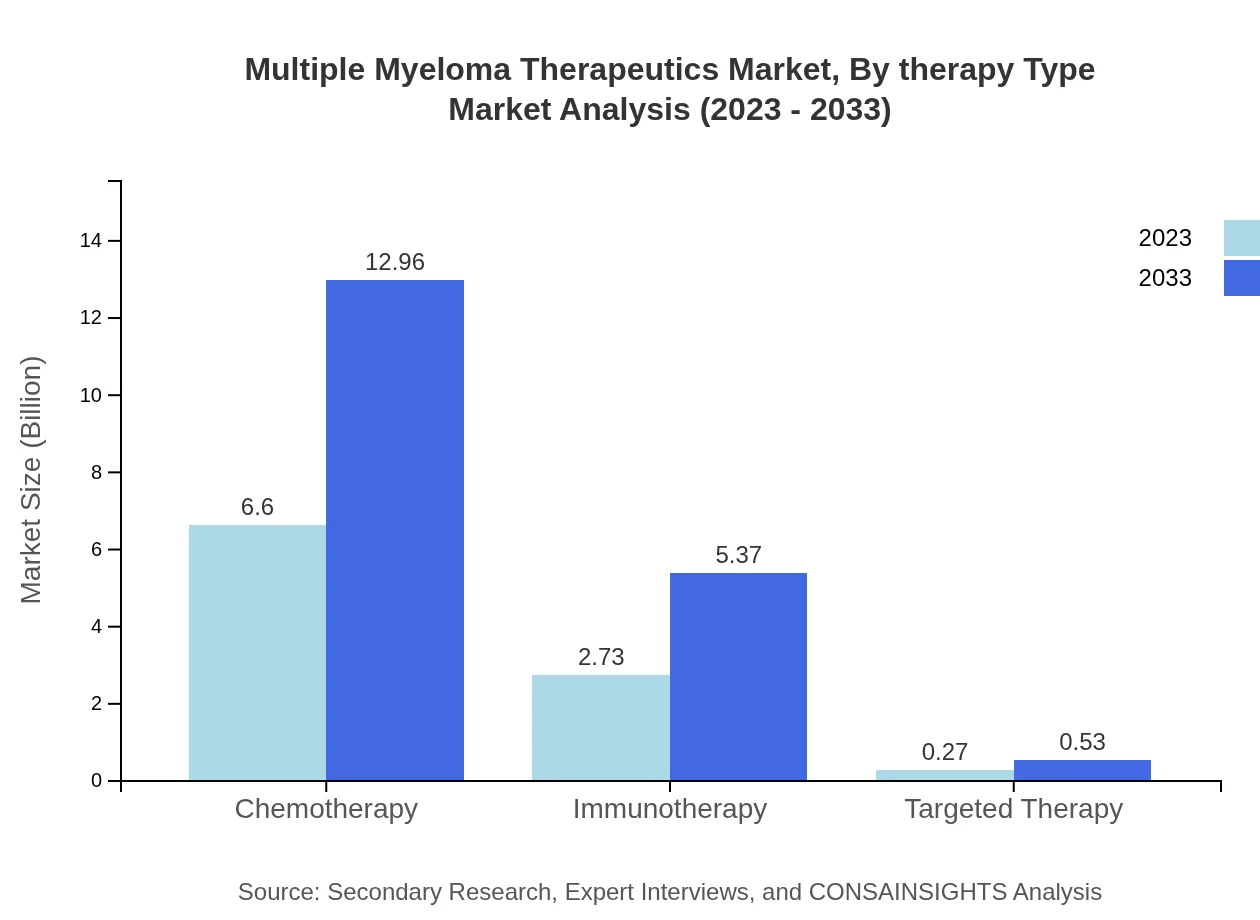
Multiple Myeloma Therapeutics Market Analysis By Therapy Type
In 2023, the chemotherapy segment was valued at $6.60 billion, holding approximately 68.7% market share, and is projected to grow to $12.96 billion by 2033. Immunotherapy, valued at $2.73 billion (28.48% share), is expected to reach $5.37 billion. Targeted therapy, while smaller, showcases potential growth from $0.27 billion to $0.53 billion.
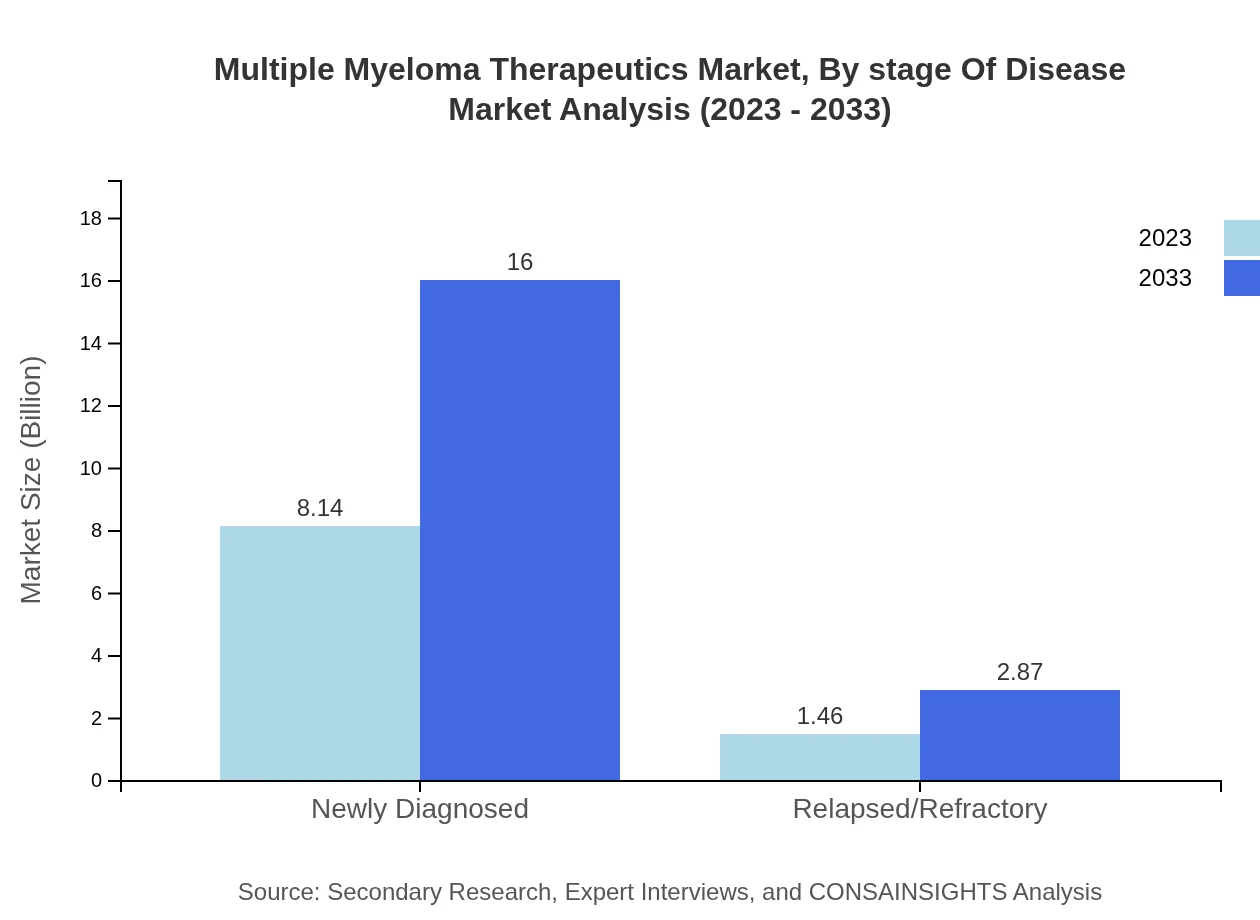
Multiple Myeloma Therapeutics Market Analysis By Stage Of Disease
The newly diagnosed segment accounts for the majority share, starting at $8.14 billion and expected to reach $16.00 billion by 2033 (84.8% share). The relapsed/refractory segment, currently at $1.46 billion, is anticipated to grow to $2.87 billion, reflecting a growing need for innovative treatment options.
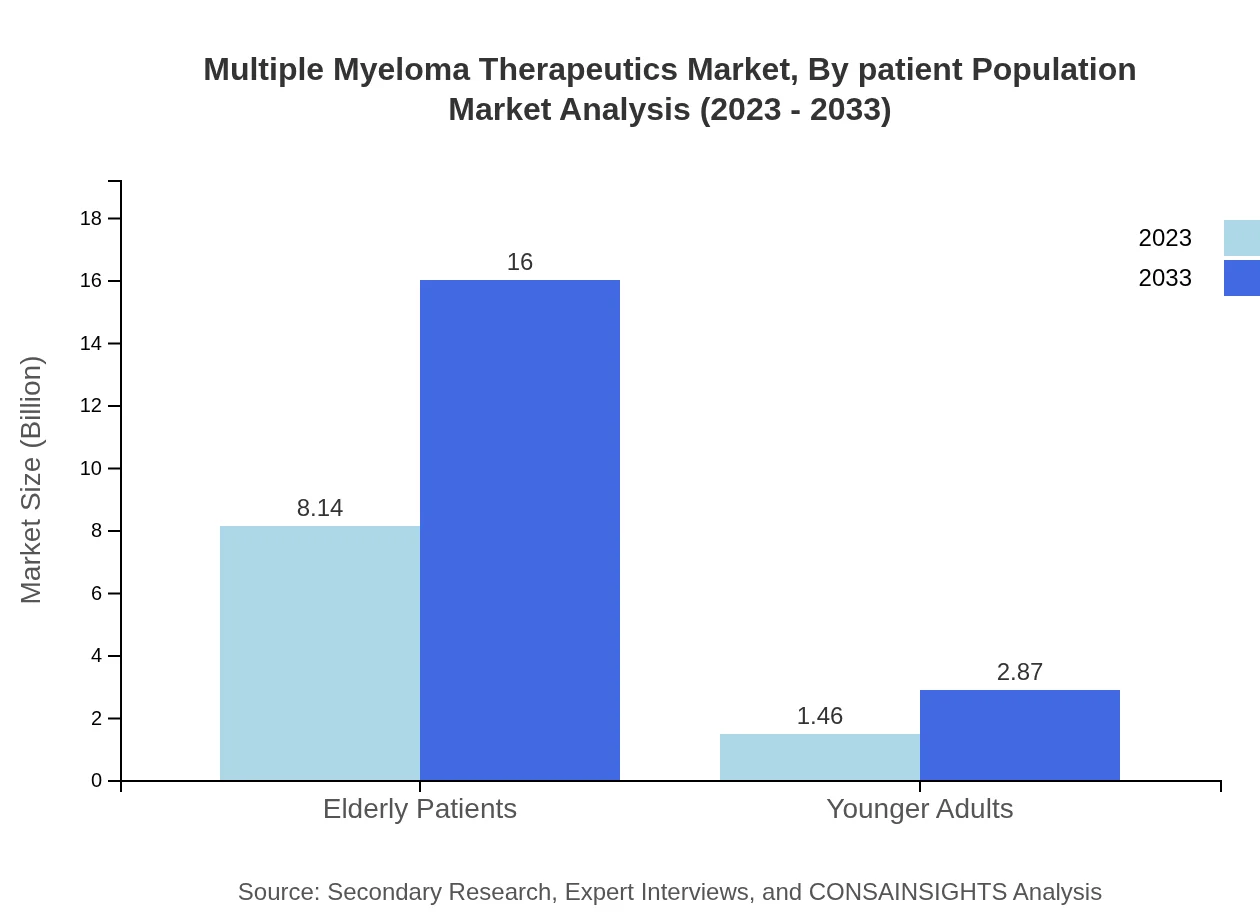
Multiple Myeloma Therapeutics Market Analysis By Patient Population
The market for elderly patients is expected to remain significant, commencing at $8.14 billion and reaching $16.00 billion by 2033 (84.8% share). Younger adults account for $1.46 billion and are projected to grow to $2.87 billion, indicating increasing therapeutic focus on younger demographics.
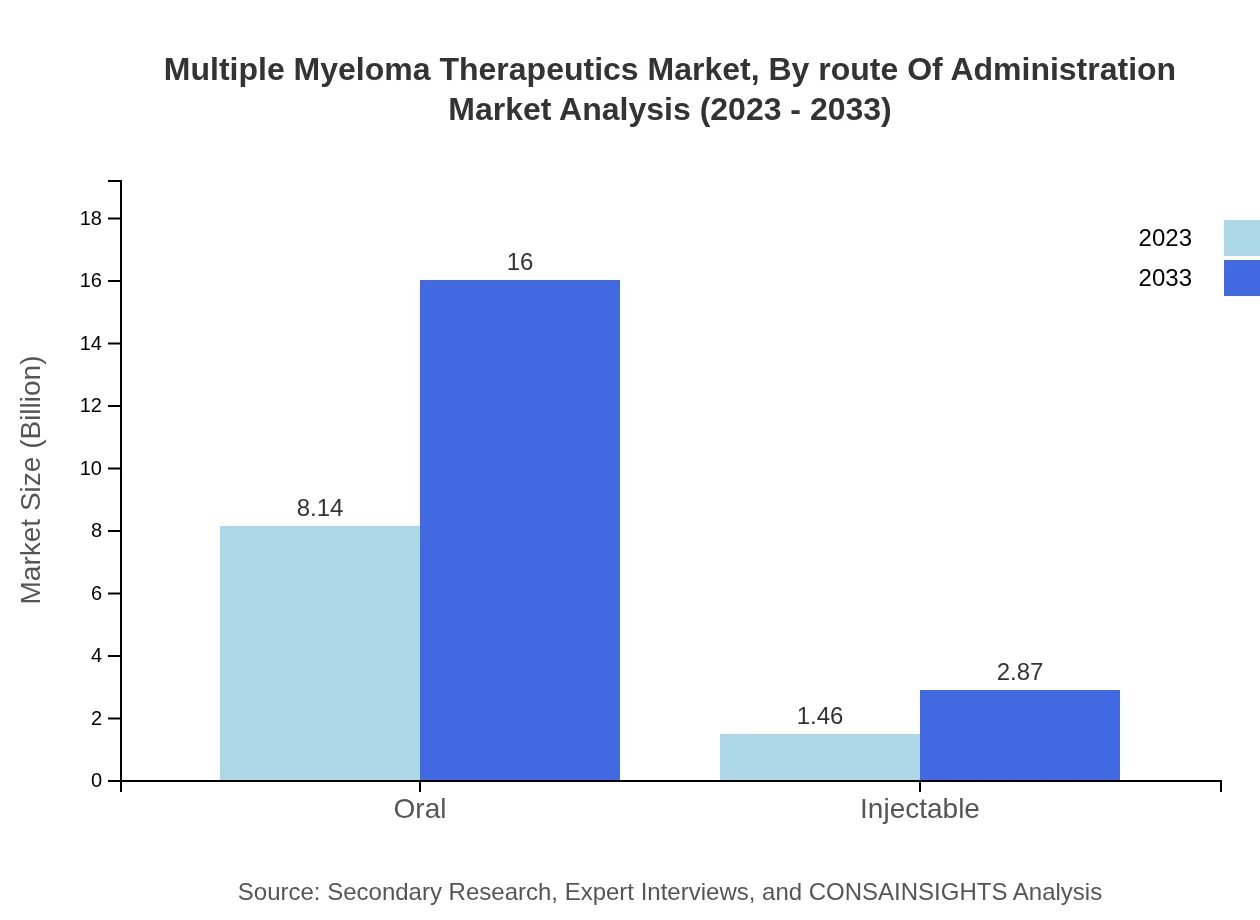
Multiple Myeloma Therapeutics Market Analysis By Route Of Administration
In 2023, oral administration dominated the market at $8.14 billion (84.8% share), expected to grow to $16.00 billion. Injectable routes, beginning at $1.46 billion (15.2% share), are projected to reach $2.87 billion, representing advancements in delivery methods.
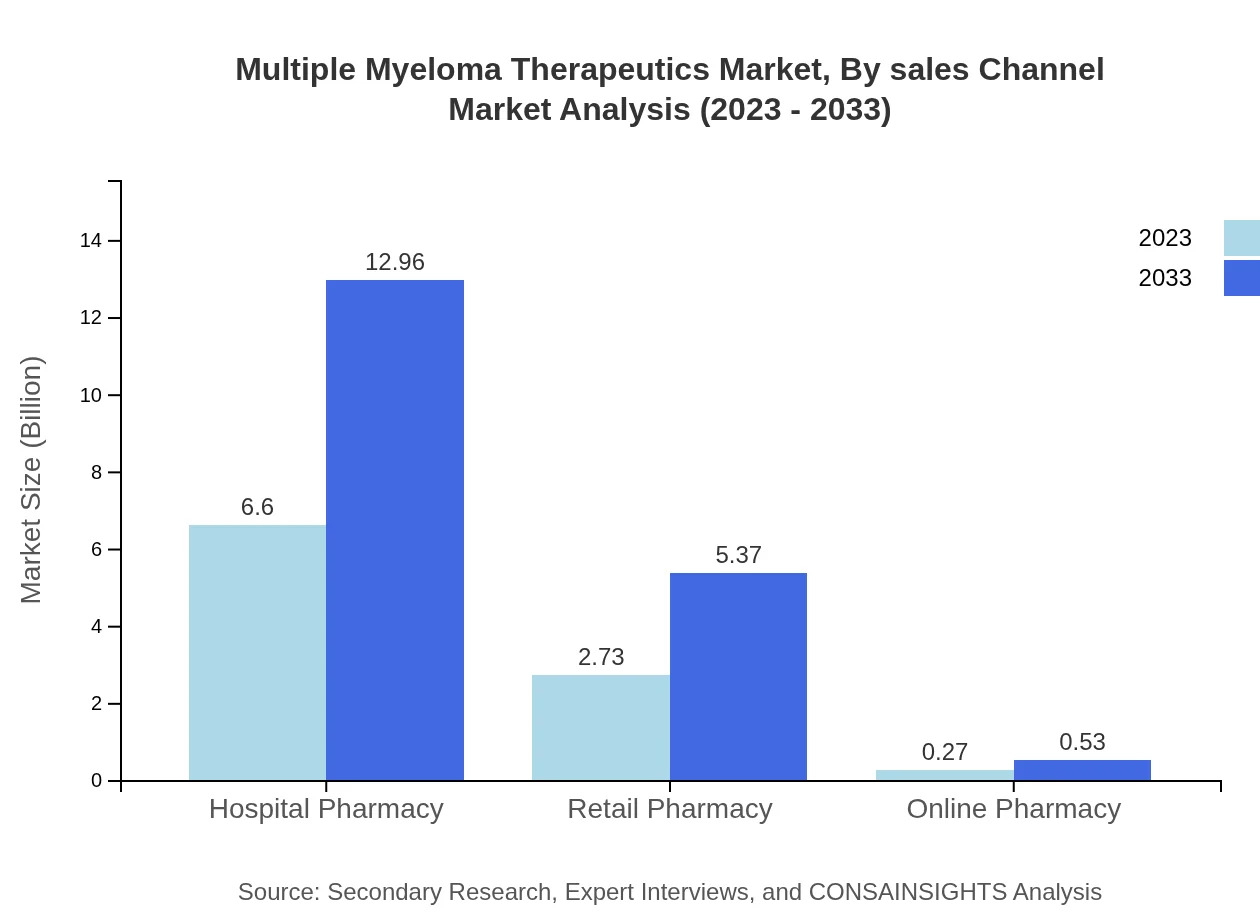
Multiple Myeloma Therapeutics Market Analysis By Sales Channel
Hospital pharmacies are leading the sales channel, starting at $6.60 billion (68.7% share), growing to $12.96 billion by 2033. Retail pharmacies constitute a significant segment with $2.73 billion (28.48% share) and are expected to rise to $5.37 billion. Online pharmacies, though smaller, show potential growth from $0.27 billion to $0.53 billion as digital health initiatives gain traction.
Multiple Myeloma Therapeutics Market Trends and Future Forecast
Tell us your focus area and get a customized research report.
Global Market Leaders and Top Companies in Multiple Myeloma Therapeutics Industry
Celgene Corporation:
A prominent player in the oncology space known for its innovative therapies for Multiple Myeloma, especially through its flagship drug, Revlimid.Johnson & Johnson:
Through its subsidiary Janssen Pharmaceuticals, it has made significant contributions with therapies like Darzalex that target multiple myeloma directly.Amgen Inc.:
Amgen is recognized for its deep commitment to advancing cancer care, particularly with therapies like Blincyto and other novel treatments.AbbVie Inc.:
AbbVie’s acquisition of AstraZeneca’s drug Imbruvica has positioned it as a leader in the Multiple Myeloma segment, significantly impacting treatment paradigms.Bristol-Myers Squibb:
Acquired Celgene and has integrated valuable therapies into its portfolio, focusing on treatment advancements in Multiple Myeloma.We're grateful to work with incredible clients.









FAQs
What is the market size of multiple Myeloma Therapeutics?
The multiple myeloma therapeutics market is projected to grow significantly, reaching a market size of $9.6 billion by 2033. The expected compound annual growth rate (CAGR) for this sector is 6.8%, driven by rising incidences and advancements in treatment options.
What are the key market players or companies in this multiple Myeloma Therapeutics industry?
Key players in the multiple myeloma therapeutics market include major pharmaceutical companies and biotechnology firms that are engaged in research, development, and commercialization of innovative therapies. These companies are pivotal in driving the market forward.
What are the primary factors driving the growth in the multiple Myeloma Therapeutics industry?
The growth of the multiple myeloma therapeutics market is primarily driven by the increasing prevalence of multiple myeloma, advancements in targeted therapies, and favorable reimbursement policies that encourage the adoption of innovative treatments.
Which region is the fastest Growing in the multiple Myeloma Therapeutics?
The fastest-growing region in the multiple myeloma therapeutics market is North America. Projections indicate a market growth from $3.59 billion in 2023 to $7.05 billion by 2033, reflecting robust investment in research and healthcare infrastructure.
Does ConsaInsights provide customized market report data for the multiple Myeloma Therapeutics industry?
Yes, ConsaInsights offers customized market report data tailored to specific interests within the multiple myeloma therapeutics industry, providing detailed insights that align with business goals and market demands.
What deliverables can I expect from this multiple Myeloma Therapeutics market research project?
Deliverables from the multiple myeloma therapeutics market research project include comprehensive reports, regional analysis, competitive landscape assessments, and detailed market forecasts, equipping stakeholders with strategic insights.
What are the market trends of multiple Myeloma Therapeutics?
Emerging trends in the multiple myeloma therapeutics market include an increased focus on personalized medicine, advancements in immunotherapy, and a shift toward combination therapies, all aiming to enhance patient outcomes and treatment efficacy.