Myasthenia Gravis Therapeutics Market Report
Published Date: 31 January 2026 | Report Code: myasthenia-gravis-therapeutics
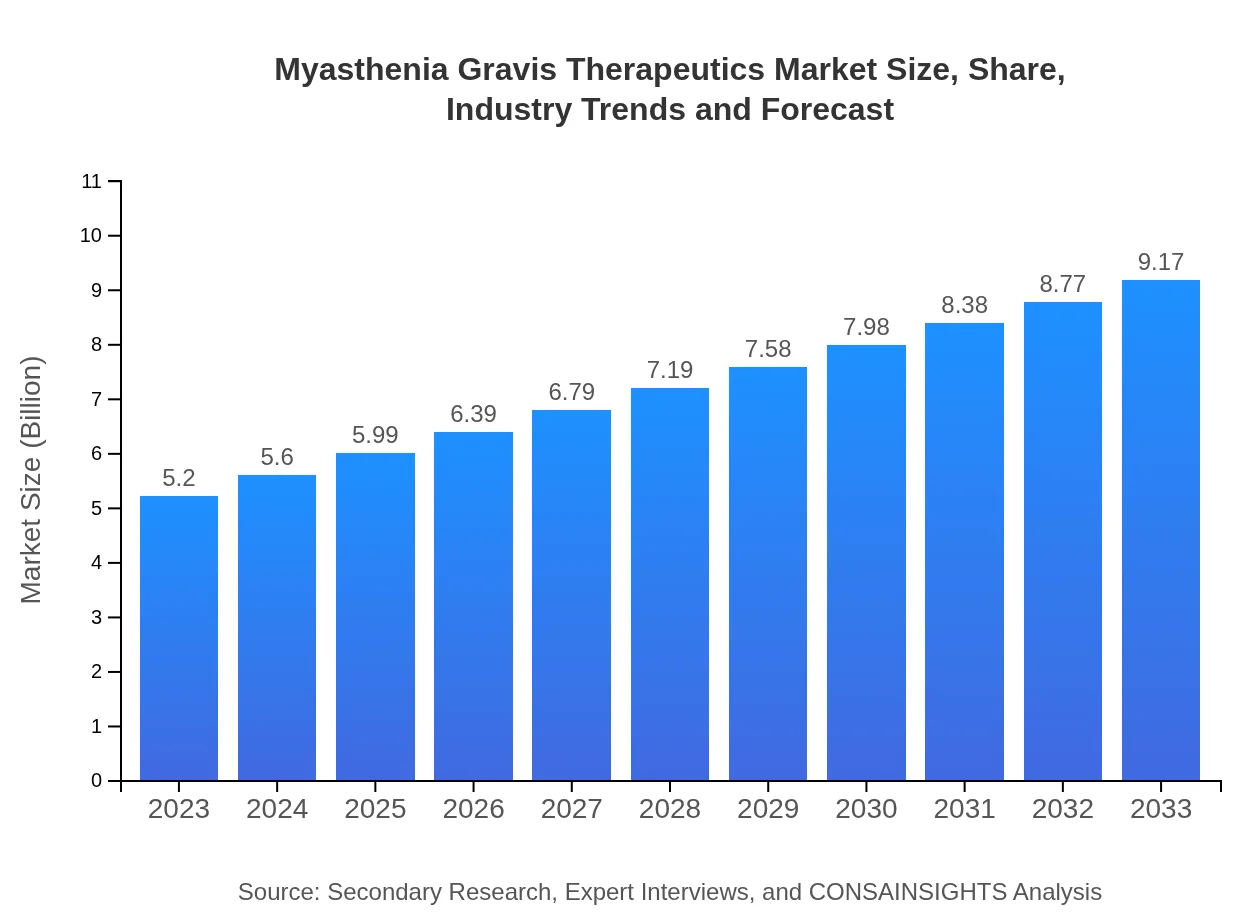
Myasthenia Gravis Therapeutics Market Size, Share, Industry Trends and Forecast to 2033
This report provides an in-depth analysis of the Myasthenia Gravis therapeutics market, focusing on key trends, market sizing, and segmentation from 2023 to 2033. Insights include regional performance, technology advancements, and profiles of leading companies in the industry.
| Metric | Value |
|---|---|
| Study Period | 2023 - 2033 |
| 2023 Market Size | $5.20 Billion |
| CAGR (2023-2033) | 5.7% |
| 2033 Market Size | $9.17 Billion |
| Top Companies | Roche Holding AG, Bristol-Myers Squibb, Novartis AG |
| Last Modified Date | 31 January 2026 |
Myasthenia Gravis Therapeutics Market Overview
Customize Myasthenia Gravis Therapeutics Market Report market research report
- ✔ Get in-depth analysis of Myasthenia Gravis Therapeutics market size, growth, and forecasts.
- ✔ Understand Myasthenia Gravis Therapeutics's regional dynamics and industry-specific trends.
- ✔ Identify potential applications, end-user demand, and growth segments in Myasthenia Gravis Therapeutics
What is the Market Size & CAGR of Myasthenia Gravis Therapeutics market in 2023?
Myasthenia Gravis Therapeutics Industry Analysis
Myasthenia Gravis Therapeutics Market Segmentation and Scope
Tell us your focus area and get a customized research report.
Myasthenia Gravis Therapeutics Market Analysis Report by Region
Europe Myasthenia Gravis Therapeutics Market Report:
Europe’s market is projected to grow from $1.42 billion in 2023 to $2.50 billion by 2033. Rising R&D investments in biological therapies and strong regulatory frameworks are critical factors supporting this growth.Asia Pacific Myasthenia Gravis Therapeutics Market Report:
The Asia Pacific region holds a market value of $1.07 billion in 2023, expected to grow to $1.88 billion by 2033, indicating a CAGR of around 6.1%. The growth is facilitated by increasing healthcare expenditures and a rising patient population seeking treatment.North America Myasthenia Gravis Therapeutics Market Report:
North America dominates the Myasthenia Gravis therapeutics market, valued at $1.75 billion in 2023 and anticipated to expand to $3.09 billion by 2033. This region's growth is propelled by advanced treatment options and substantial investment in healthcare.South America Myasthenia Gravis Therapeutics Market Report:
South America is projected to increase from $0.46 billion in 2023 to $0.81 billion in 2033, with growth attributes linked to improving healthcare infrastructure and emerging awareness of Myasthenia Gravis.Middle East & Africa Myasthenia Gravis Therapeutics Market Report:
The Middle East and Africa market is expected to increase from $0.50 billion in 2023 to $0.89 billion in 2033. Greater access to novel therapies and healthcare improvement are driving this market.Tell us your focus area and get a customized research report.
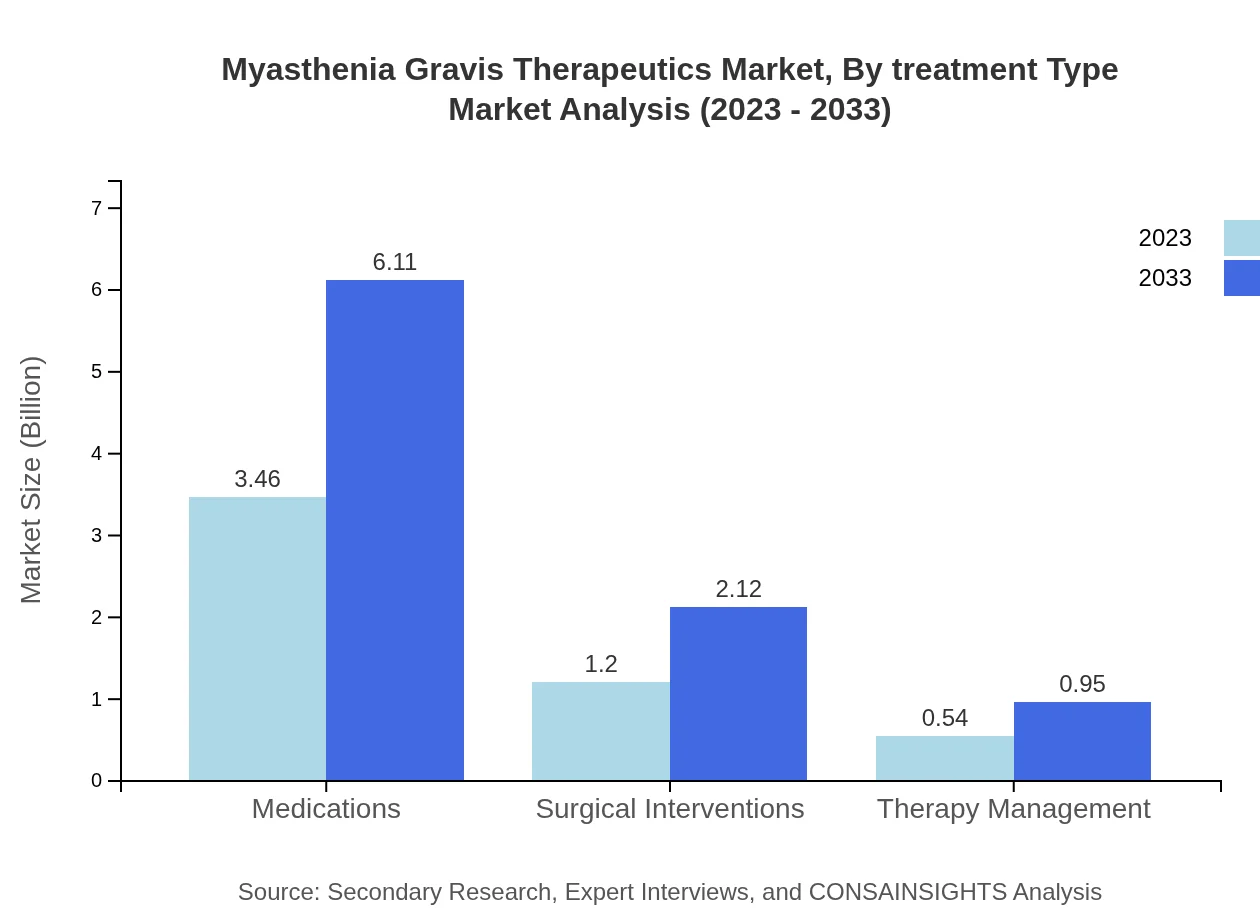
Myasthenia Gravis Therapeutics Market Analysis By Treatment Type
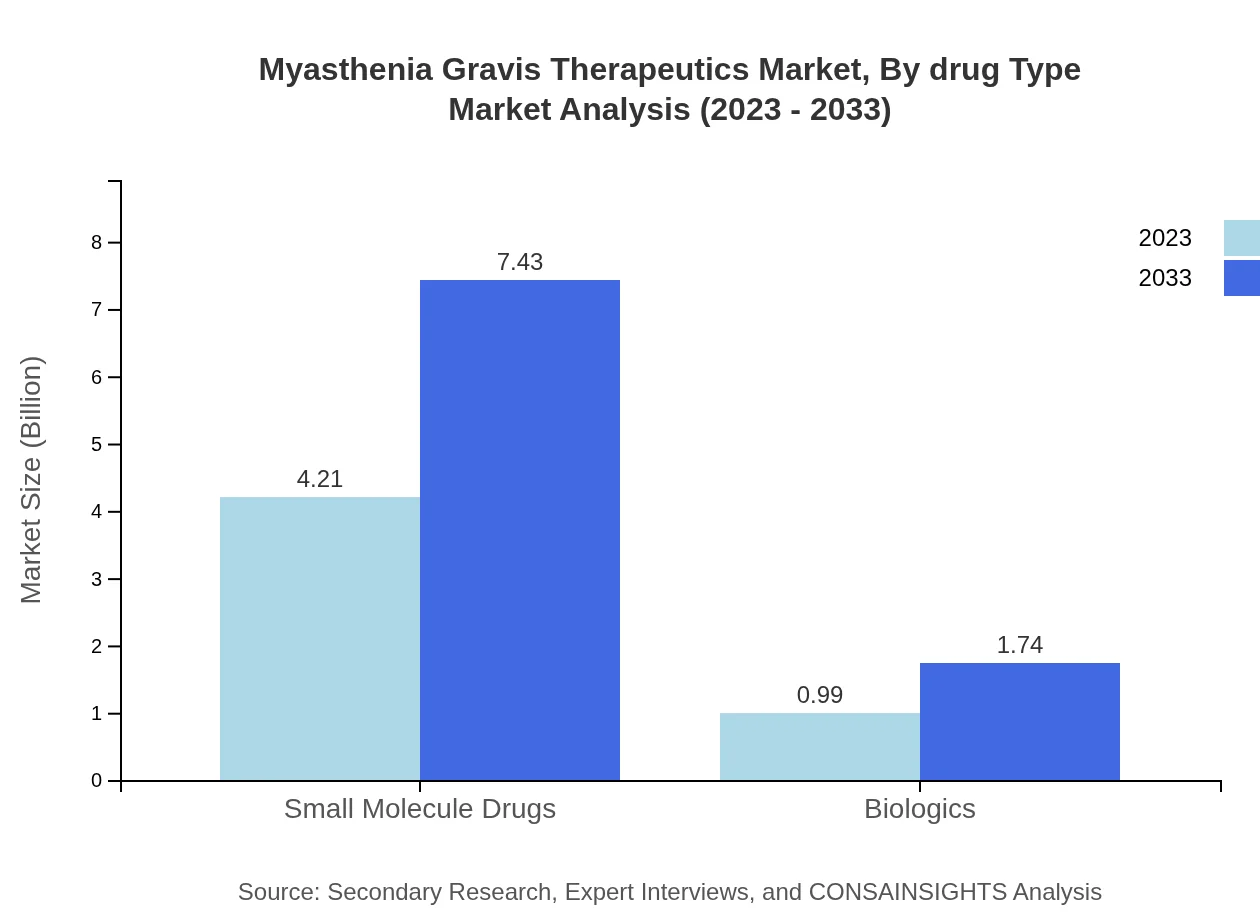
In 2023, the market size for treatment types includes small molecules at $4.21 billion, accounting for 80.98% market share, and biologics at $0.99 billion, with 19.02%. By 2033, small molecule therapies will grow to $7.43 billion, maintaining the majority share, while biologics will reach $1.74 billion.
Myasthenia Gravis Therapeutics Market Analysis By Drug Type
Market performances for medications are robust, with a size of $3.46 billion in 2023, retained until 2033 due to steady demand. Surgical interventions will grow from $1.20 billion to $2.12 billion, sustaining their essential role in treatment.
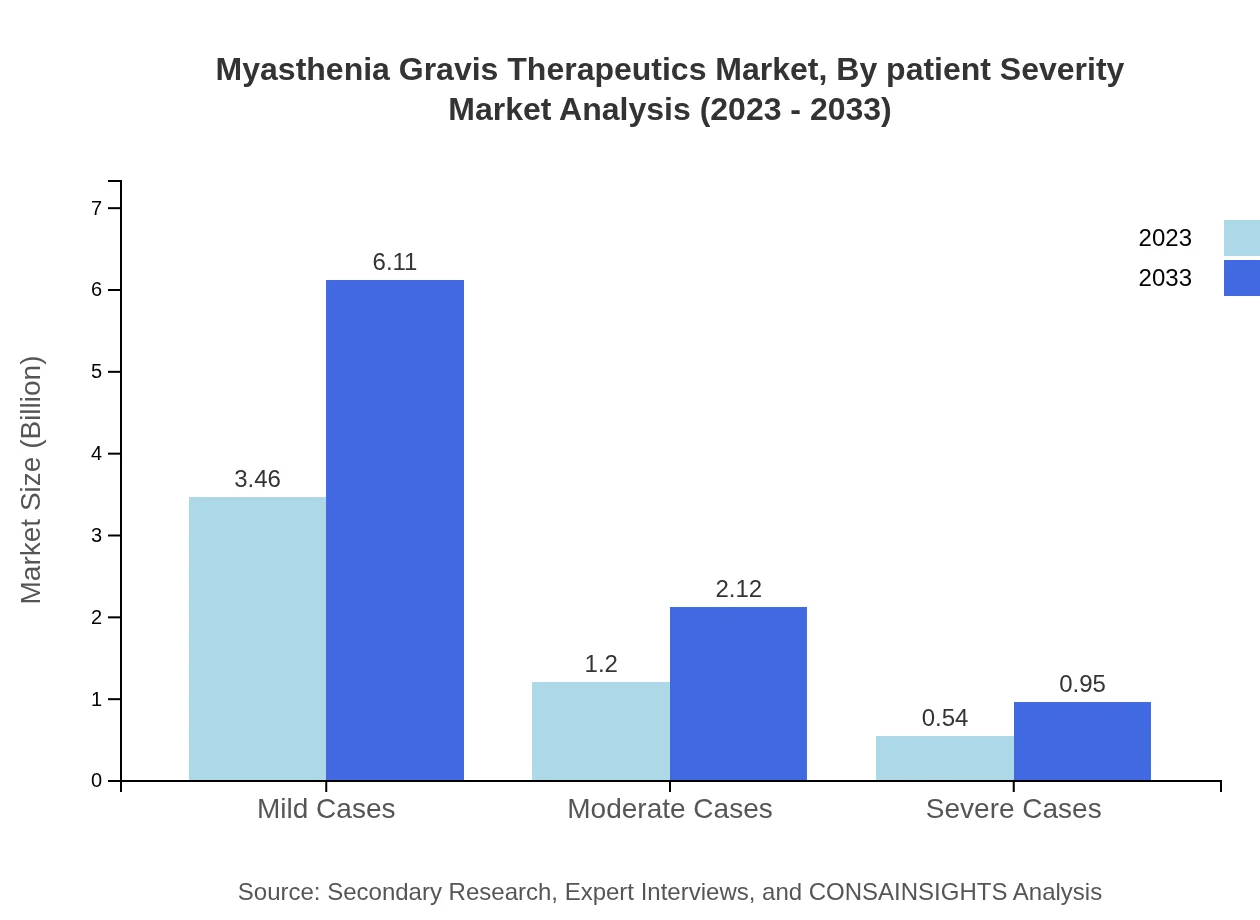
Myasthenia Gravis Therapeutics Market Analysis By Patient Severity
The market size for mild cases is projected to grow from $3.46 billion to $6.11 billion, while moderate and severe cases will see incremental growth as well, reflecting evolving treatment strategies tailored to patient needs.
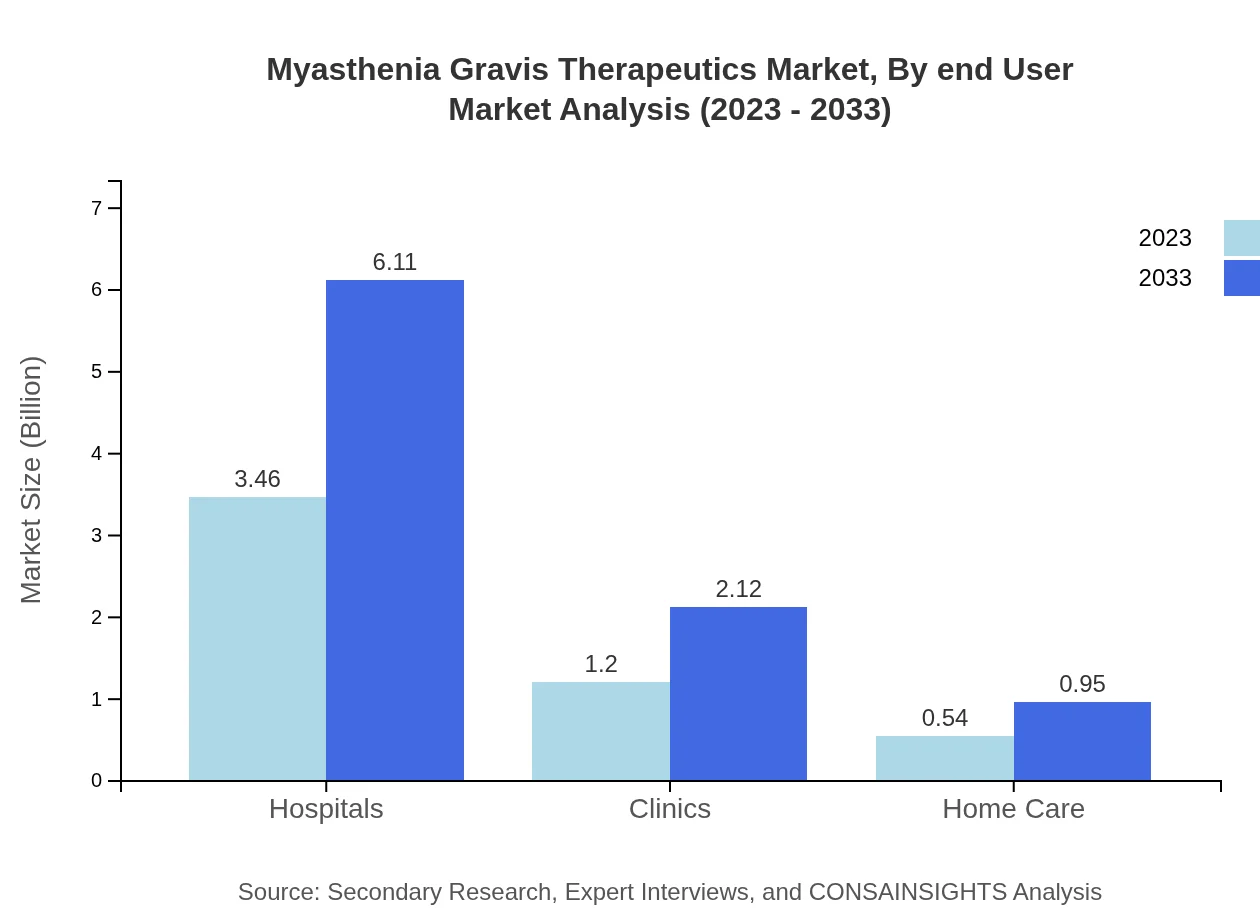
Myasthenia Gravis Therapeutics Market Analysis By End User
Hospitals lead the market with a size of $3.46 billion in 2023, expected to rise to $6.11 billion by 2033, reflecting a consistent need for specialized care. Clinics and home care also play pivotal roles in patient management.
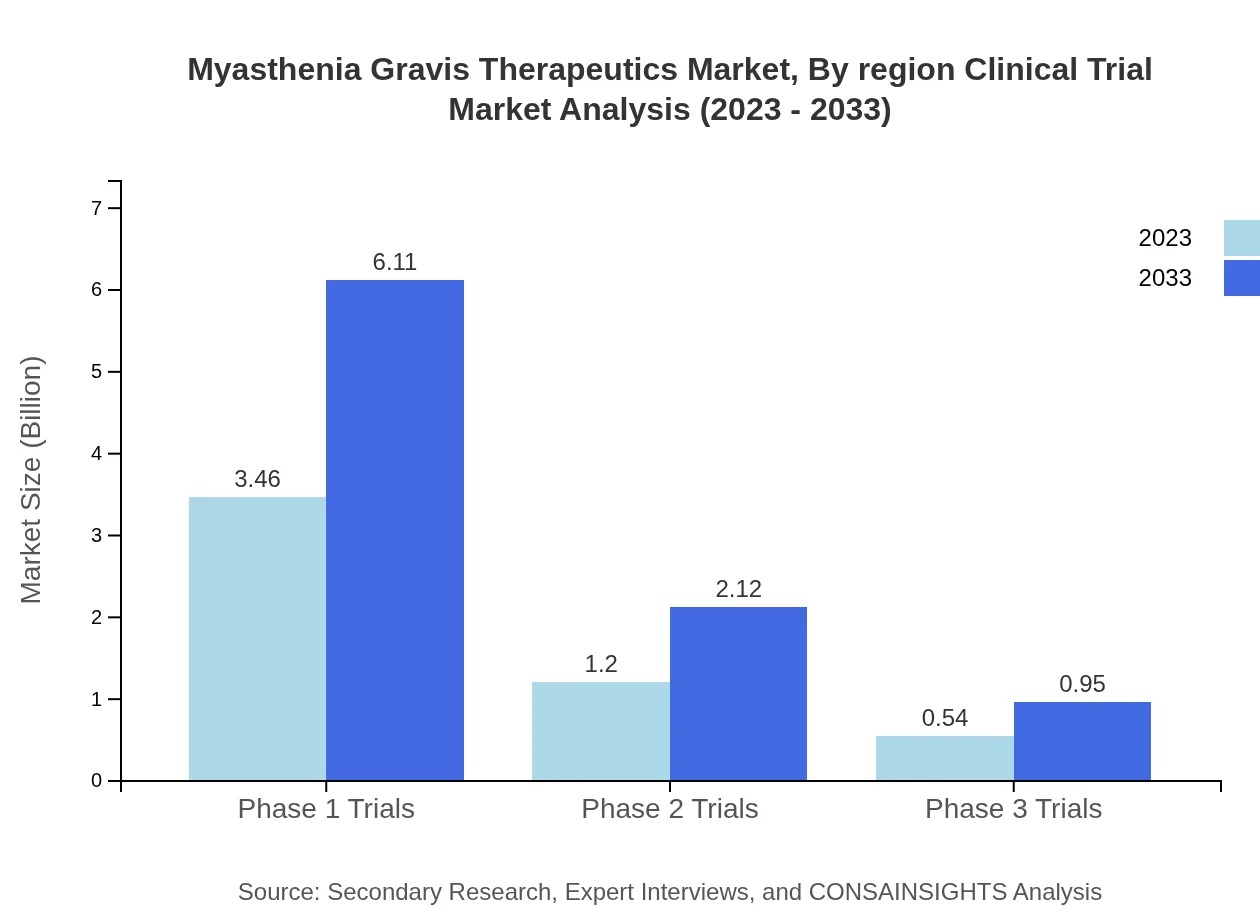
Myasthenia Gravis Therapeutics Market Analysis By Region Clinical Trial
The clinical trial market shows promise, with Phase 1 trials dominating at $3.46 billion in 2023 and predicted growth to $6.11 billion by 2033. Ongoing advances and new entries continue to renew interest in clinical research for MG therapies.
Myasthenia Gravis Therapeutics Market Trends and Future Forecast
Tell us your focus area and get a customized research report.
Global Market Leaders and Top Companies in Myasthenia Gravis Therapeutics Industry
Roche Holding AG:
Roche is a leading biotech company recognized for its innovative treatments in autoimmune diseases, including Myasthenia Gravis. Its focus on personalized medicine bridges gaps in MG management.Bristol-Myers Squibb:
Bristol-Myers Squibb develops proteins and monoclonal antibodies that treat Myasthenia Gravis. The company's commitment to R&D fuels the advent of new therapy options.Novartis AG:
Novartis specializes in high-impact medical solutions to treat MG. Its extensive pipeline reflects advancements in therapeutic techniques that improve patient quality of life.We're grateful to work with incredible clients.









FAQs
What is the market size of myasthenia Gravis Therapeutics?
The myasthenia gravis therapeutics market is valued at approximately $5.2 billion in 2023, with a projected compound annual growth rate (CAGR) of 5.7% through 2033, indicating significant growth and demand for innovative treatment options in this segment.
What are the key market players or companies in this myasthenia Gravis Therapeutics industry?
Key players in the myasthenia gravis therapeutics market include major pharmaceutical companies and biotech firms specializing in neurology. These companies focus on developing innovative therapies, including small molecule drugs and biologics, creating a competitive landscape essential for future advancements.
What are the primary factors driving the growth in the myasthenia Gravis Therapeutics industry?
Growth in the myasthenia gravis therapeutics sector is driven by rising prevalence of the disease, advancements in treatment technologies, increasing research efforts, and growing awareness among healthcare professionals regarding effective management strategies for patients.
Which region is the fastest Growing in the myasthenia Gravis Therapeutics?
The North American region is the fastest-growing market for myasthenia gravis therapeutics, expected to grow from $1.75 billion in 2023 to $3.09 billion by 2033. Europe and Asia Pacific are also expanding significantly, indicating global demand.
Does ConsaInsights provide customized market report data for the myasthenia Gravis Therapeutics industry?
Yes, ConsaInsights offers customized market report data tailored to specific requirements in the myasthenia gravis therapeutics industry. Clients can gain insights tailored to their strategic needs and market conditions.
What deliverables can I expect from this myasthenia Gravis Therapeutics market research project?
Expect comprehensive deliverables including detailed market analysis reports, insights on key trends, competitive landscape assessments, segment analysis, and forecasts to support strategic decision-making in the myasthenia gravis therapeutics market.
What are the market trends of myasthenia Gravis Therapeutics?
Trends in the myasthenia gravis therapeutics market include increasing focus on biologic therapies, growth in home care services, and a shift towards personalized medicine approaches, which are transforming treatment protocols and enhancing patient outcomes.