Mycoplasma Testing Market Report
Published Date: 31 January 2026 | Report Code: mycoplasma-testing
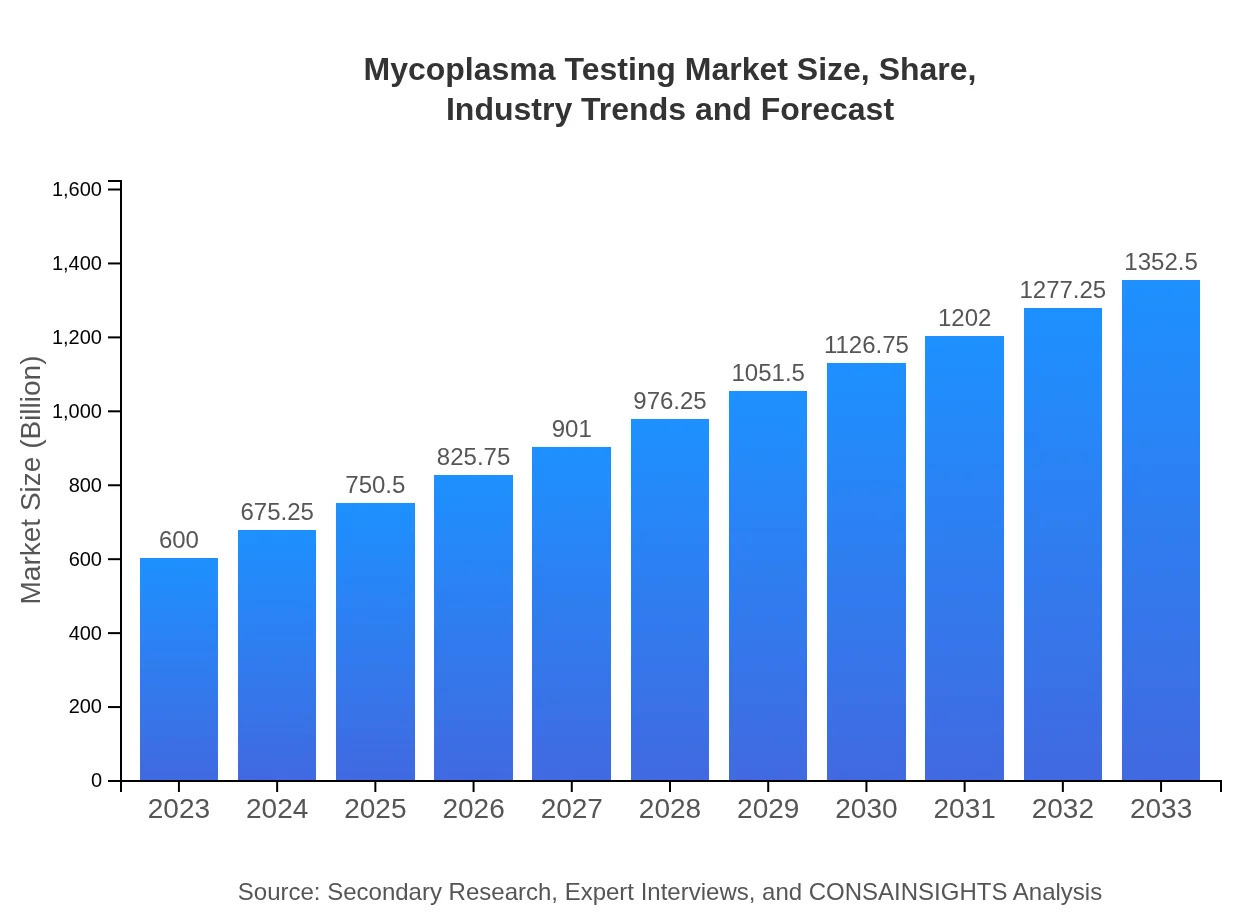
Mycoplasma Testing Market Size, Share, Industry Trends and Forecast to 2033
This report explores the Mycoplasma Testing market, providing insights into market trends, size, technological advancements, and regional analysis from 2023 to 2033.
| Metric | Value |
|---|---|
| Study Period | 2023 - 2033 |
| 2023 Market Size | $600.00 Million |
| CAGR (2023-2033) | 8.2% |
| 2033 Market Size | $1352.50 Million |
| Top Companies | Thermo Fisher Scientific, Merck KGaA, Lonza Group, Bio-Rad Laboratories |
| Last Modified Date | 31 January 2026 |
Mycoplasma Testing Market Overview
Customize Mycoplasma Testing Market Report market research report
- ✔ Get in-depth analysis of Mycoplasma Testing market size, growth, and forecasts.
- ✔ Understand Mycoplasma Testing's regional dynamics and industry-specific trends.
- ✔ Identify potential applications, end-user demand, and growth segments in Mycoplasma Testing
What is the Market Size & CAGR of Mycoplasma Testing market in 2033?
Mycoplasma Testing Industry Analysis
Mycoplasma Testing Market Segmentation and Scope
Tell us your focus area and get a customized research report.
Mycoplasma Testing Market Analysis Report by Region
Europe Mycoplasma Testing Market Report:
In Europe, the Mycoplasma Testing market's value is anticipated to grow from $147.48 million in 2023 to $332.44 million by 2033. The growth stems from stringent regulations mandating mycoplasma testing in manufacturing processes of biologics and a flourishing biotechnology industry. Countries like Germany and the UK are spearheading growth due to their advanced healthcare systems and robust research infrastructure.Asia Pacific Mycoplasma Testing Market Report:
The Asia Pacific region is seeing a substantial rise in the Mycoplasma Testing market, estimated to grow from $127.44 million in 2023 to $287.27 million by 2033. The growth is driven by increasing investments in pharmaceutical research and development, particularly in countries like China and India, and an expanding life sciences sector. Moreover, the growing awareness of mycoplasma contamination risks among researchers and manufacturers is propelling demand for efficient testing methods.North America Mycoplasma Testing Market Report:
North America is the leading market for Mycoplasma Testing, with an estimated value of $225.90 million in 2023, projected to reach $509.22 million by 2033. The strong presence of key market players, coupled with extensive regulatory requirements in the pharmaceutical sector, drives the demand for accurate mycoplasma detection. Furthermore, ongoing research activities and increased funding for biotechnology contribute significantly to regional market expansion.South America Mycoplasma Testing Market Report:
In South America, the Mycoplasma Testing market is anticipated to grow from $27.30 million in 2023 to $61.54 million by 2033. The region is gradually adopting advanced testing technologies, supported by a rise in biopharmaceutical industry activities. Efforts by local governments to enhance healthcare infrastructure are also aiding the growth of this market segment.Middle East & Africa Mycoplasma Testing Market Report:
The Middle East and Africa region are expected to witness growth in the Mycoplasma Testing market from $71.88 million in 2023 to $162.03 million by 2033. Increased investment in biomedical research and development, along with rising collaborations between local and international biotechnology firms, is enhancing the market landscape. Additionally, the growing healthcare expenditure and need for high-quality diagnostic tools are influencing market growth positively.Tell us your focus area and get a customized research report.
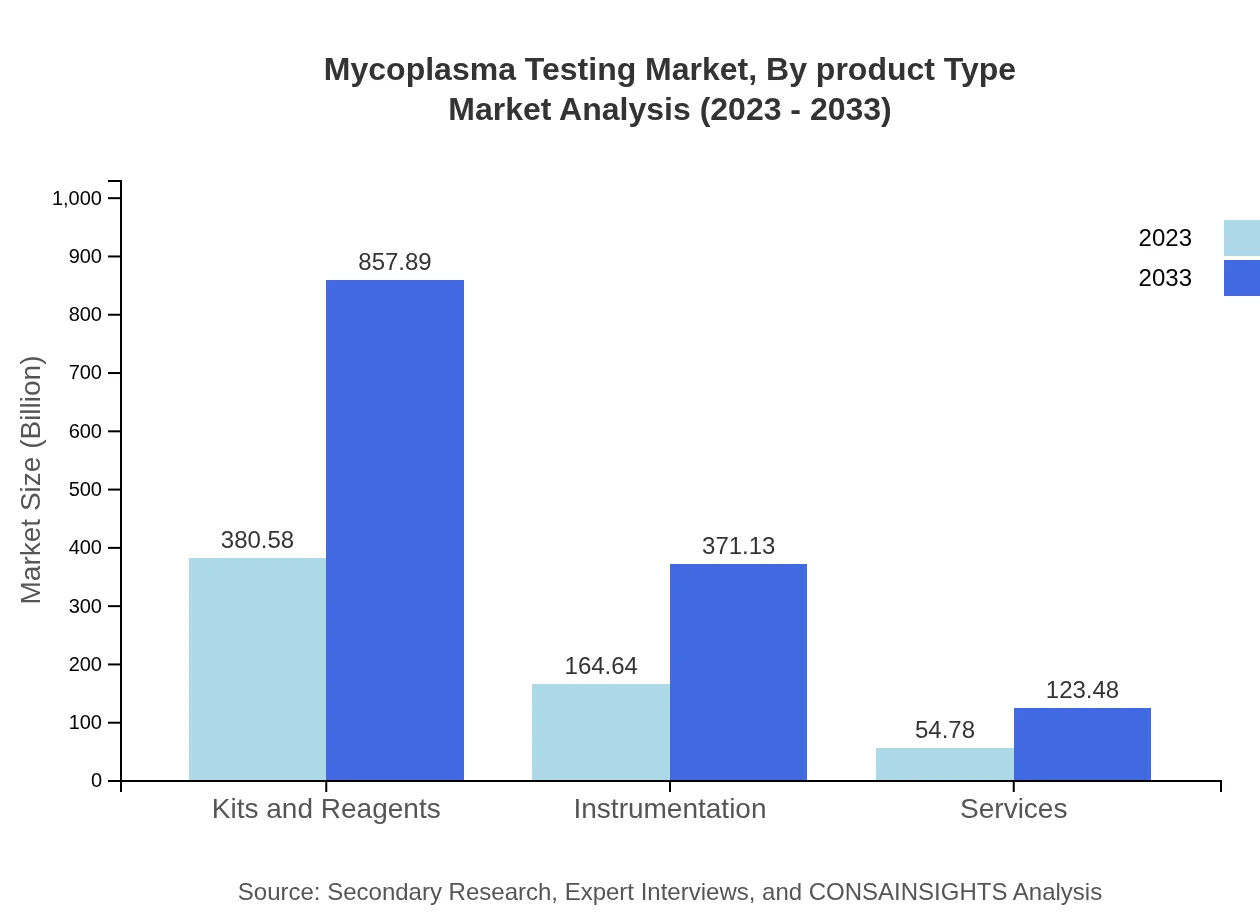
Mycoplasma Testing Market Analysis By Product Type
The Mycoplasma Testing market is primarily driven by various product types including Kits and Reagents, which are projected to grow from $380.58 million in 2023 to $857.89 million by 2033. Instrumentation is set to rise from $164.64 million to $371.13 million during the same period, while Services are expected to grow from $54.78 million to $123.48 million. The increasing demand for reliable kits and reagents due to their vital role in mycoplasma detection underscores their significance as a market segment.
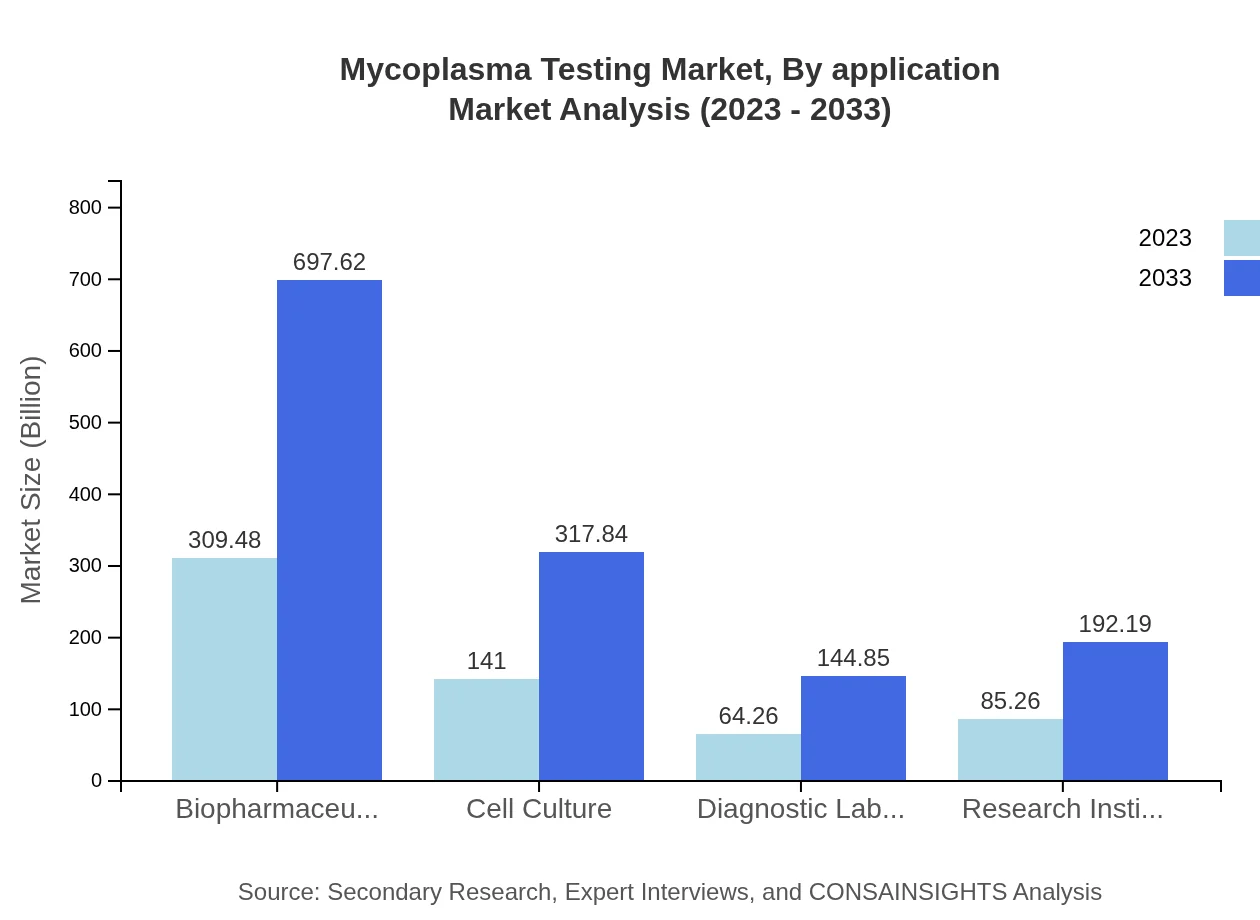
Mycoplasma Testing Market Analysis By Application
Applications in the Mycoplasma Testing market include Biopharmaceuticals, Academic Research, and Diagnostics. The pharmaceutical application dominates the market, driven by strict regulatory compliance requirements and expanding production capabilities in biologics, with projected growth from $309.48 million in 2023 to $697.62 million by 2033. Academic institutions also contribute significantly, with needs for testing in various research settings, reinforcing the importance of this segment.
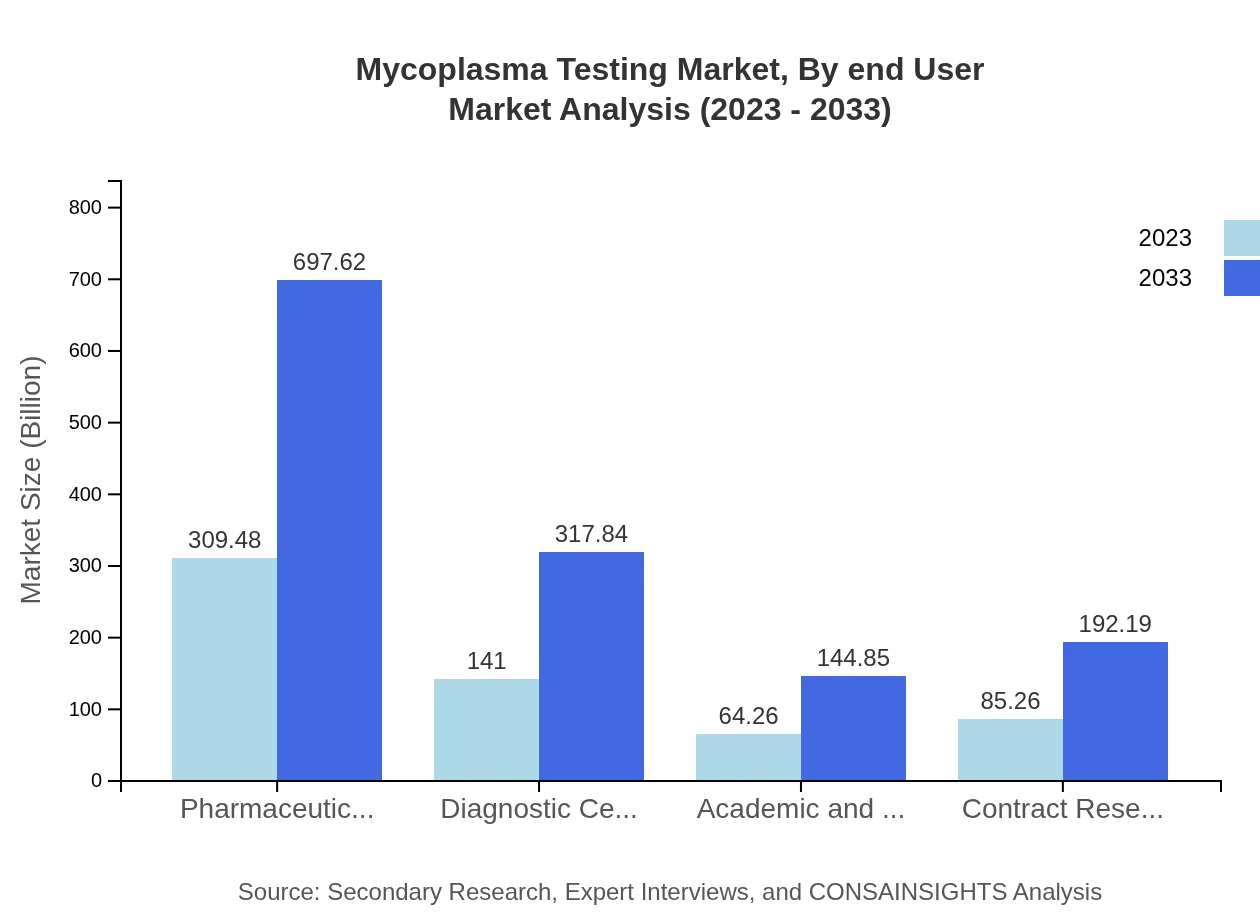
Mycoplasma Testing Market Analysis By End User
End-users of Mycoplasma Testing services include Pharmaceutical Companies, Diagnostic Centers, and Academic Institutions. Pharmaceutical companies hold a substantial market share, valued at $309.48 million in 2023 and expected to reach $697.62 million by 2033. Diagnostic centers and academic institutions are also essential contributors, with respective forecasts of $141 million to $317.84 million and $64.26 million to $144.85 million over the same period.
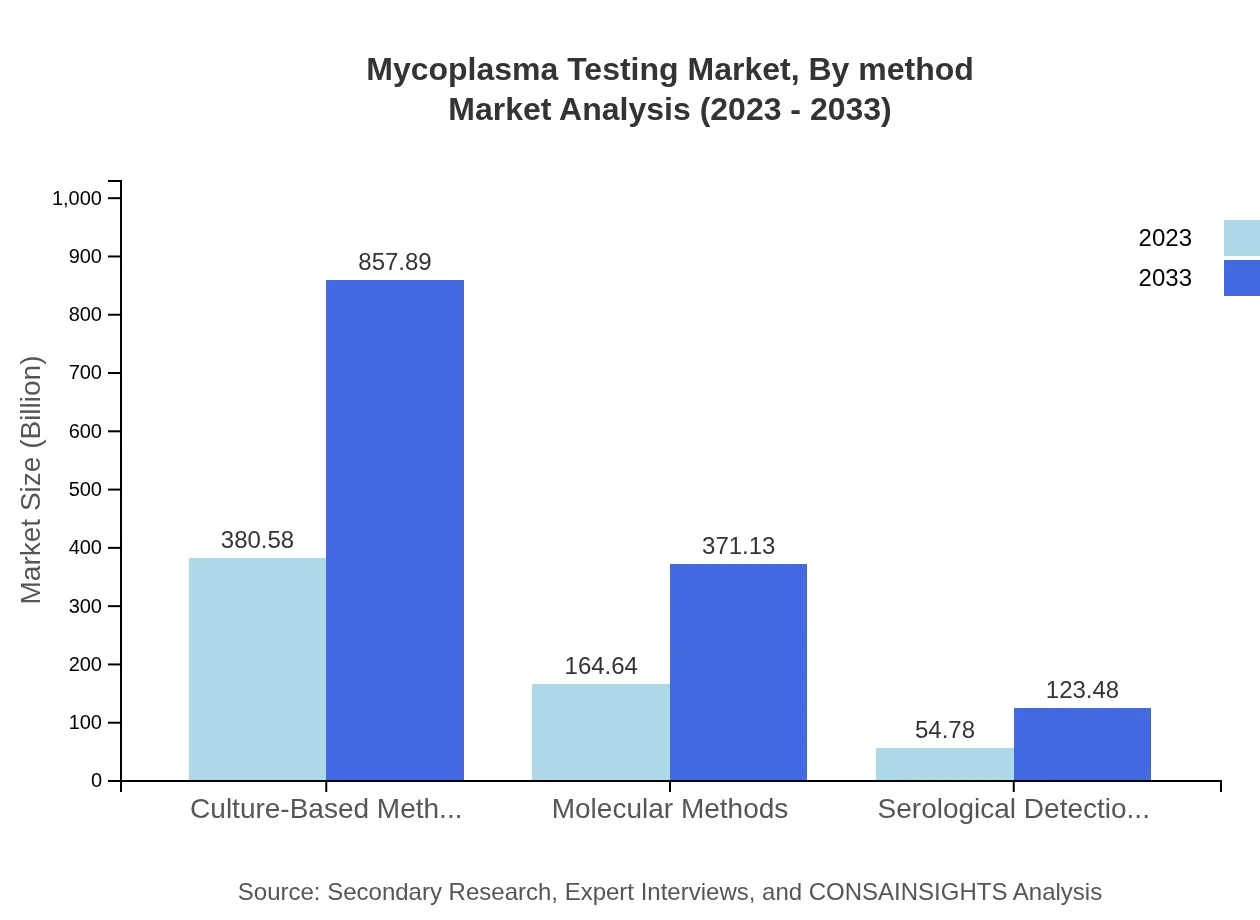
Mycoplasma Testing Market Analysis By Method
The market is divided based on testing methods such as Culture-Based Methods, Molecular Methods, and Serological Detection. Culture-Based Methods are anticipated to maintain a dominant position, increasing from $380.58 million to $857.89 million. Molecular Methods are projected to grow from $164.64 million to $371.13 million while Serological Detection Methods are expected to evolve from $54.78 million to reach $123.48 million. These methods underpin the varied testing needs across different research and clinical applications.
Mycoplasma Testing Market Trends and Future Forecast
Tell us your focus area and get a customized research report.
Global Market Leaders and Top Companies in Mycoplasma Testing Industry
Thermo Fisher Scientific:
A leading provider of scientific instrumentation, reagents, and software services for laboratories, known for their advanced mycoplasma testing solutions and diagnostics.Merck KGaA:
A global leader in life science products, offering comprehensive mycoplasma testing kits and services for biopharmaceutical and research applications.Lonza Group:
Specializes in biologics manufacturing, providing mycoplasma testing solutions integral to the cell and gene therapy sector.Bio-Rad Laboratories:
Known for its innovative diagnostic products, Bio-Rad offers key mycoplasma testing kits essential for the quality control in biomanufacturing.We're grateful to work with incredible clients.









FAQs
What is the market size of Mycoplasma Testing?
The global mycoplasma testing market is currently valued at approximately $600 million, with an impressive compound annual growth rate (CAGR) of 8.2%. The market is projected to show significant growth over the next decade, indicating a thriving sector in diagnostics.
What are the key market players or companies in the Mycoplasma Testing industry?
Key players in the mycoplasma testing industry typically include major pharmaceutical companies, diagnostic labs, and biotechnology firms. These entities lead market innovation and development in testing methodologies, contributing to robust growth in the sector.
What are the primary factors driving the growth in the Mycoplasma Testing industry?
Growth factors for the mycoplasma testing industry include the increasing prevalence of mycoplasma infections in patients, rising demand for quality control in biopharmaceutical manufacturing, and advancements in testing technologies that enhance detection accuracy and efficiency.
Which region is the fastest Growing in the Mycoplasma Testing market?
North America is currently the fastest-growing region in the mycoplasma testing market. Between 2023 and 2033, it’s expected to grow from $225.90 million to $509.22 million, driven by a robust healthcare infrastructure and increased R&D investments.
Does ConsaInsights provide customized market report data for the Mycoplasma Testing industry?
Yes, ConsaInsights offers customized market report data tailored to specific needs for the mycoplasma testing industry. Clients can request specific insights, trends, and analysis that align with their business objectives and market interests.
What deliverables can I expect from this Mycoplasma Testing market research project?
Deliverables from the mycoplasma testing market research project include comprehensive market analysis reports, detailed segmentation data, growth forecasts, competitive landscape insights, and actionable recommendations to inform strategic decision-making.
What are the market trends of Mycoplasma Testing?
Current trends in the mycoplasma testing market reveal an increasing shift towards molecular methods and culture-based techniques. Additionally, there’s a growing emphasis on automation and rapid testing solutions, reflecting the industry's response to changing healthcare demands.