Myelodysplastic Syndrome Mds Treatment Market Report
Published Date: 31 January 2026 | Report Code: myelodysplastic-syndrome-mds-treatment
Myelodysplastic Syndrome Mds Treatment Market Size, Share, Industry Trends and Forecast to 2033
This report provides an in-depth analysis of the Myelodysplastic Syndrome (MDS) treatment market, covering market size, growth trends, industry insights, and competitive landscapes from 2023 to 2033.
| Metric | Value |
|---|---|
| Study Period | 2023 - 2033 |
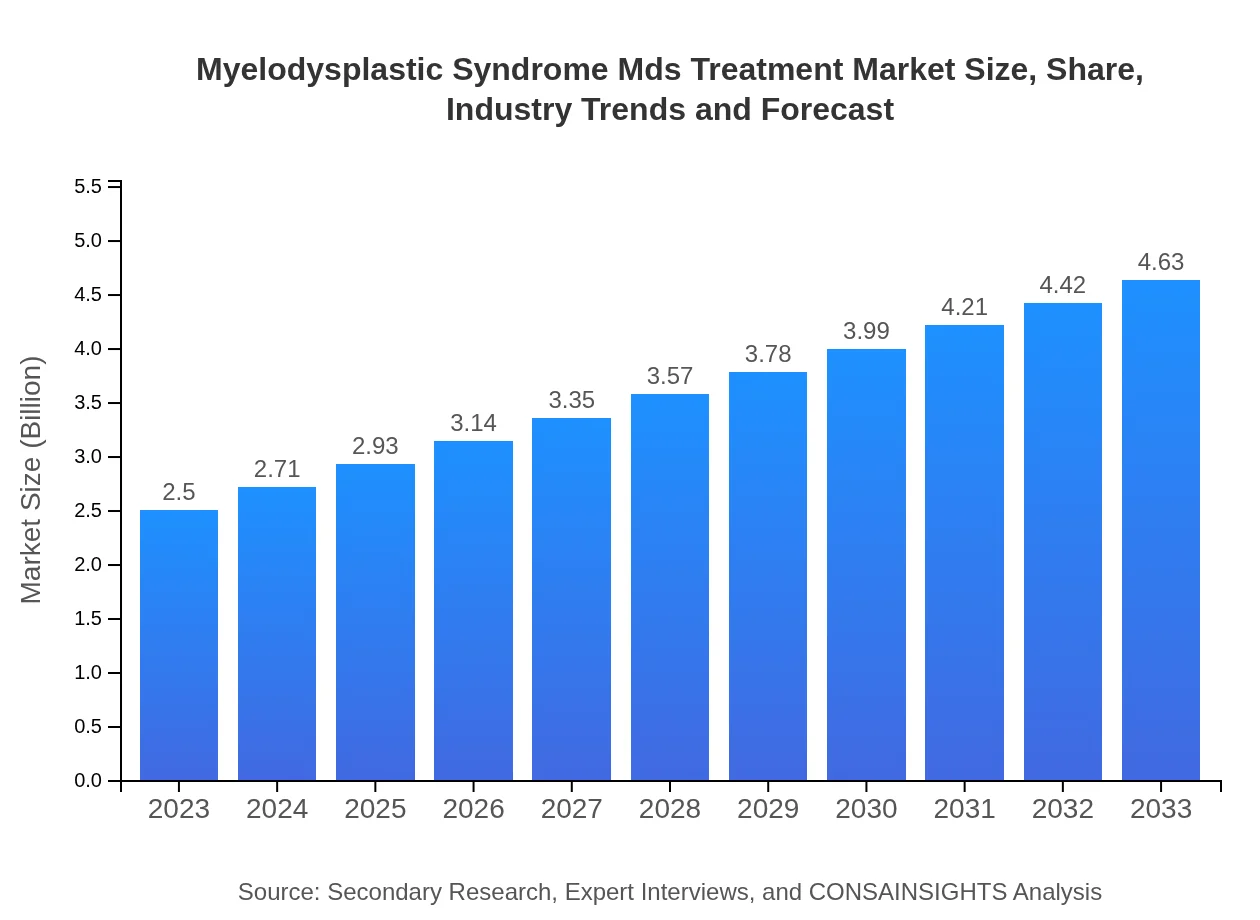
| 2023 Market Size | $2.50 Billion |
| CAGR (2023-2033) | 6.2% |
| 2033 Market Size | $4.63 Billion |
| Top Companies | Celgene Corporation, Amgen Inc., Novartis AG, Bristol-Myers Squibb, Roche Holding AG |
| Last Modified Date | 31 January 2026 |
Myelodysplastic Syndrome Mds Treatment Market Overview
Customize Myelodysplastic Syndrome Mds Treatment Market Report market research report
- ✔ Get in-depth analysis of Myelodysplastic Syndrome Mds Treatment market size, growth, and forecasts.
- ✔ Understand Myelodysplastic Syndrome Mds Treatment's regional dynamics and industry-specific trends.
- ✔ Identify potential applications, end-user demand, and growth segments in Myelodysplastic Syndrome Mds Treatment
What is the Market Size & CAGR of Myelodysplastic Syndrome Mds Treatment market in 2023?
Myelodysplastic Syndrome Mds Treatment Industry Analysis
Myelodysplastic Syndrome Mds Treatment Market Segmentation and Scope
Tell us your focus area and get a customized research report.
Myelodysplastic Syndrome Mds Treatment Market Analysis Report by Region
Europe Myelodysplastic Syndrome Mds Treatment Market Report:
The European market size is forecasted to rise from $0.62 billion in 2023 to $1.14 billion in 2033, driven by increasing government initiatives to support cancer care and a rise in MDS cases due to a growing geriatric population.Asia Pacific Myelodysplastic Syndrome Mds Treatment Market Report:
In 2023, the Asia Pacific MDS treatment market is estimated at $0.51 billion, growing to $0.94 billion by 2033, driven by increased awareness and healthcare investments. The growing prevalence of MDS in aging populations and the establishment of more treatment facilities are contributing to this growth.North America Myelodysplastic Syndrome Mds Treatment Market Report:
North America, the largest market, is projected to increase from $0.85 billion in 2023 to $1.58 billion by 2033. This is attributed to high healthcare spending, advanced treatment options, and robust research and development activities in the pharmaceutical sector.South America Myelodysplastic Syndrome Mds Treatment Market Report:
The South American market is expected to grow from $0.23 billion in 2023 to $0.43 billion in 2033. The growth is supported by expanding healthcare access and improvements in diagnostic capabilities and treatment options in the region, despite economic challenges.Middle East & Africa Myelodysplastic Syndrome Mds Treatment Market Report:
In the Middle East and Africa, the market is anticipated to grow from $0.29 billion in 2023 to $0.54 billion by 2033, facilitated by improving healthcare infrastructure and rising investments in the healthcare sector.Tell us your focus area and get a customized research report.
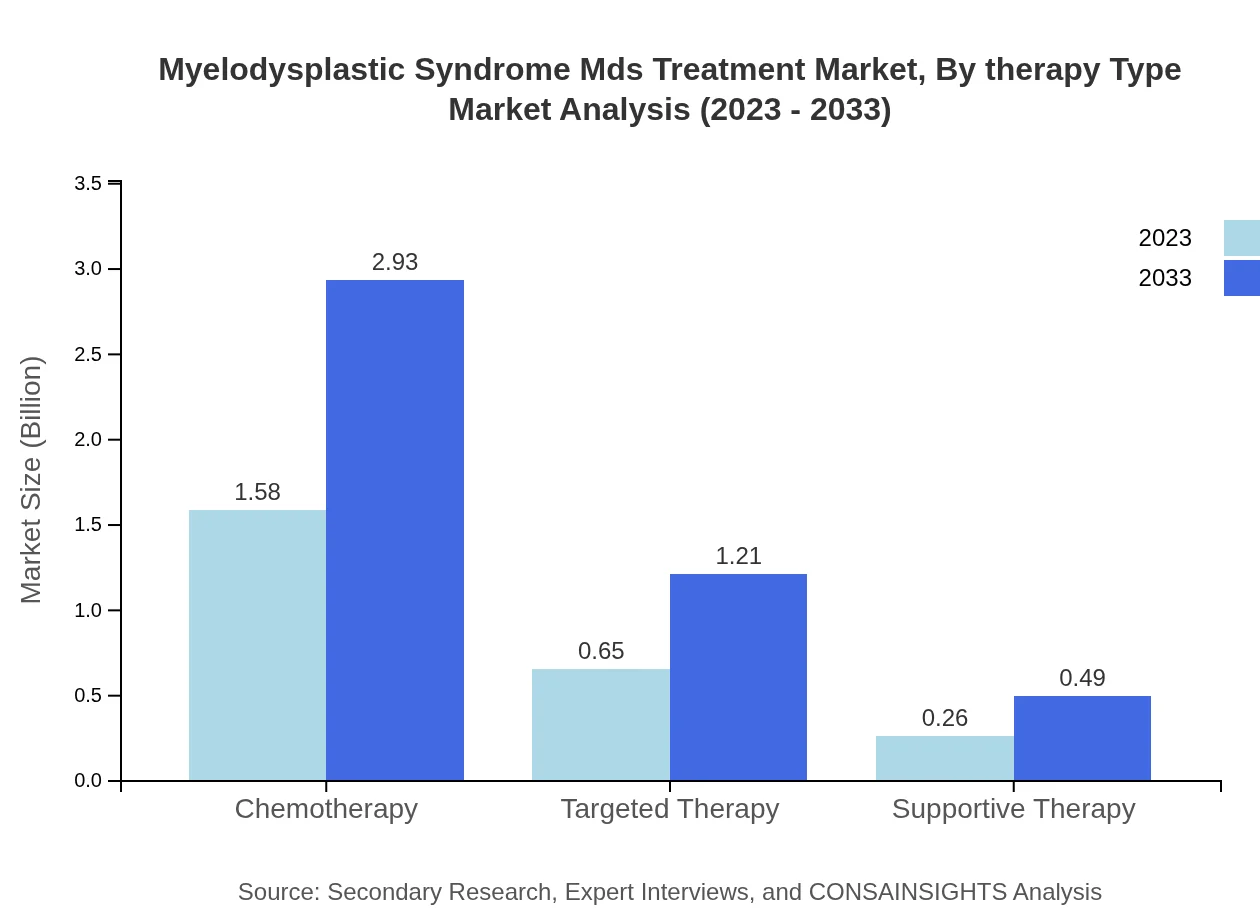
Myelodysplastic Syndrome Mds Treatment Market Analysis By Therapy Type
In 2023, chemotherapy commands a significant share of the treatment market, valued at $1.58 billion, expected to rise to $2.93 billion by 2033, maintaining approximately 63.24% of the market share. Targeted therapy, estimated at $0.65 billion in 2023, is projected to reach $1.21 billion, representing 26.17% share by 2033. Supportive therapies will grow from $0.26 billion to $0.49 billion, holding a 10.59% share.
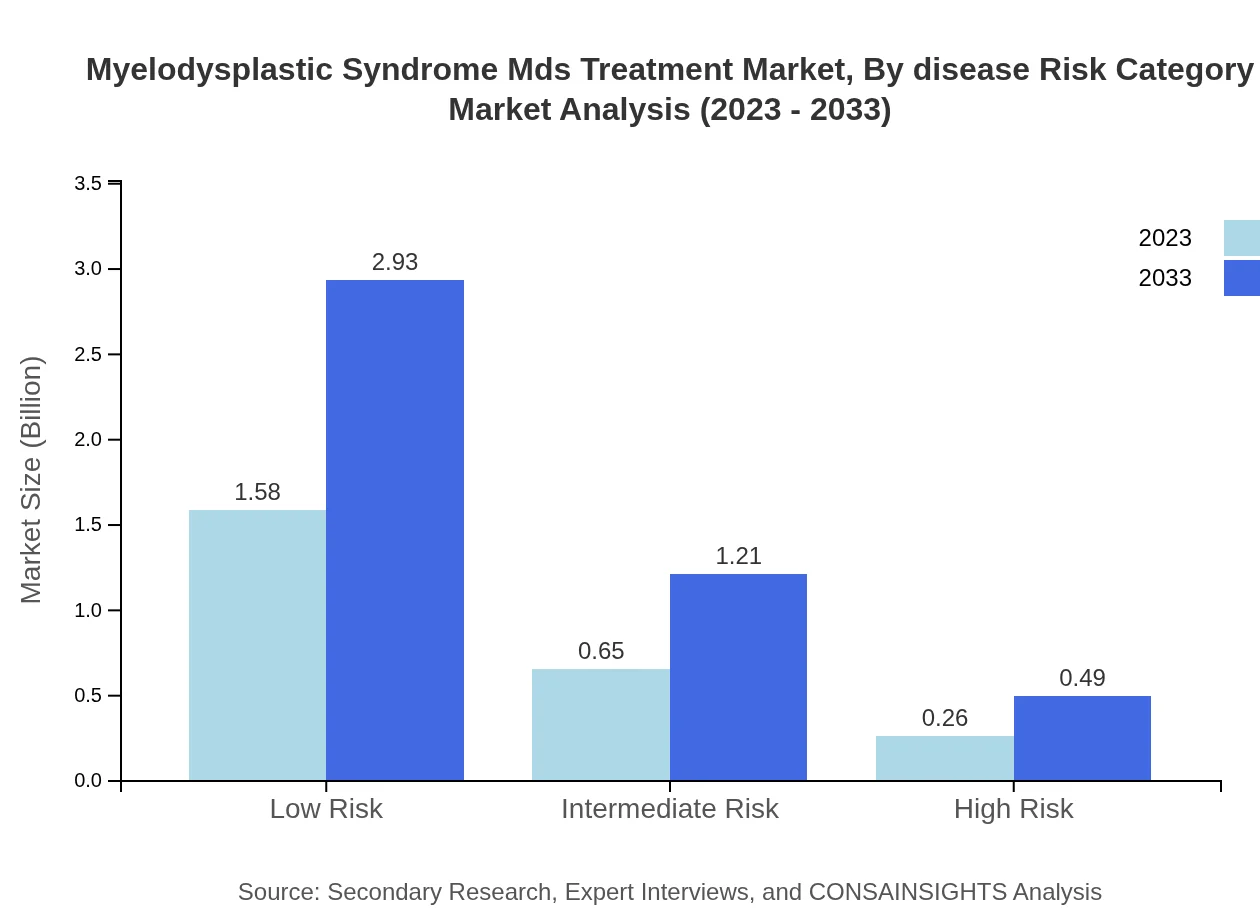
Myelodysplastic Syndrome Mds Treatment Market Analysis By Disease Risk Category
Low-risk MDS treatments, representing 63.24% of the market share, are expected to grow from $1.58 billion to $2.93 billion. Intermediate-risk therapies share, maintaining 26.17% of the market, will increase from $0.65 billion to $1.21 billion, while high-risk categories will see modest growth from $0.26 billion to $0.49 billion.
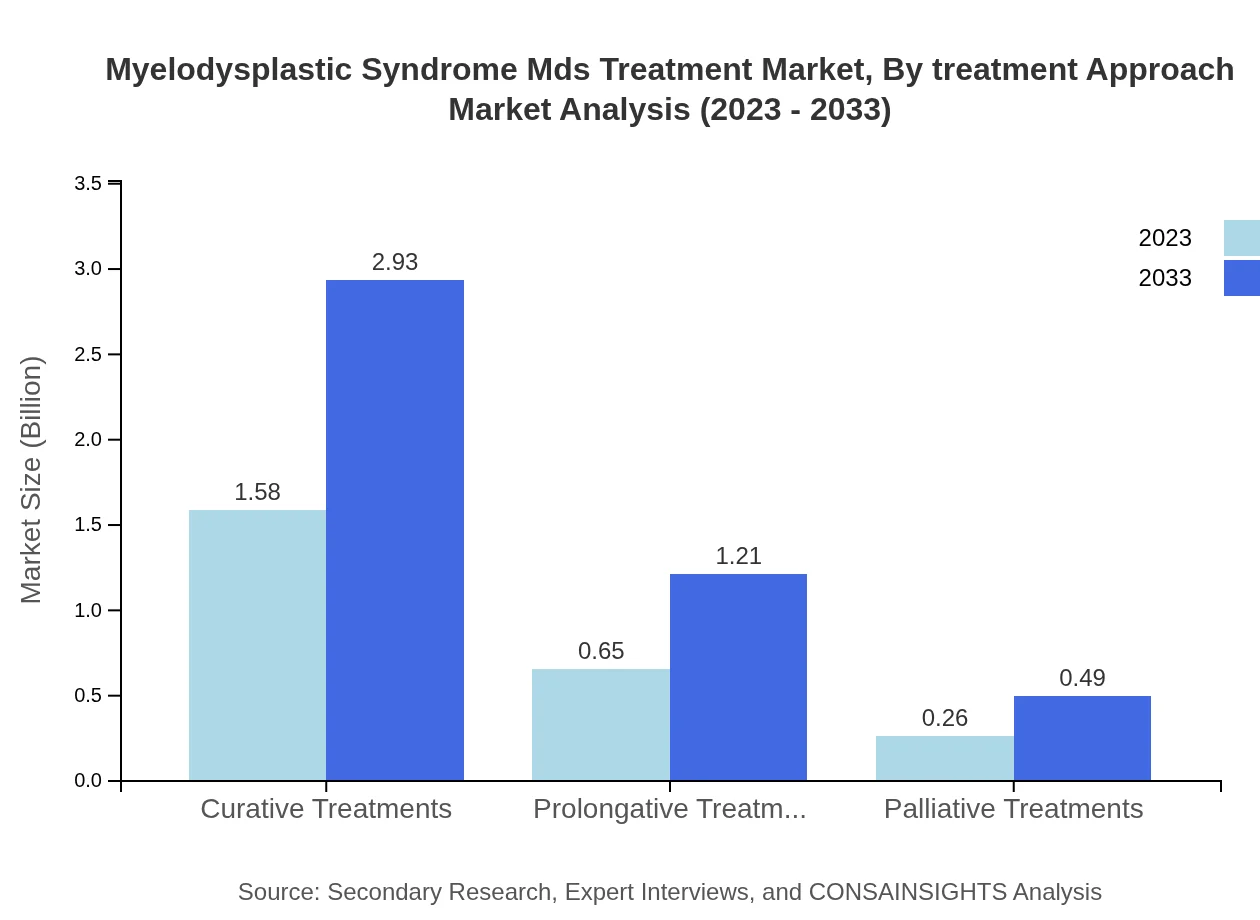
Myelodysplastic Syndrome Mds Treatment Market Analysis By Treatment Approach
First-line treatments dominate the market at 63.24% share, from $1.58 billion in 2023 to $2.93 billion by 2033. Second-line and third-line treatment approaches follow at 26.17% and 10.59% share, expected to increase from $0.65 and $0.26 billion respectively, reaching $1.21 and $0.49 billion.
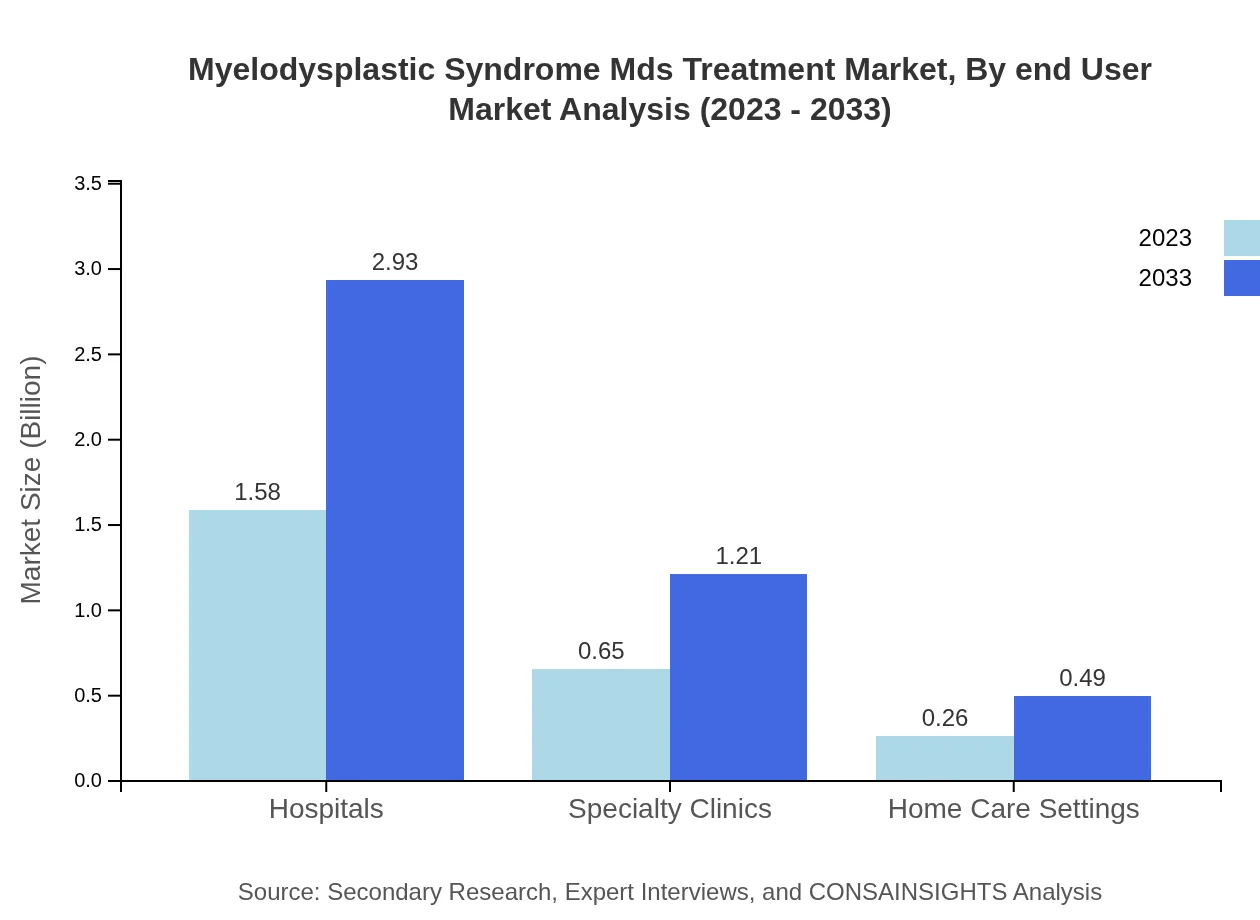
Myelodysplastic Syndrome Mds Treatment Market Analysis By End User
Hospitals continue to be the predominant end-user segment in the MDS treatment market, accounting for $1.58 billion in 2023 and projected to reach $2.93 billion by 2033. Specialty clinics are emerging as significant players, expanding from $0.65 billion to $1.21 billion. Home care settings are also growing modestly from $0.26 billion to $0.49 billion.
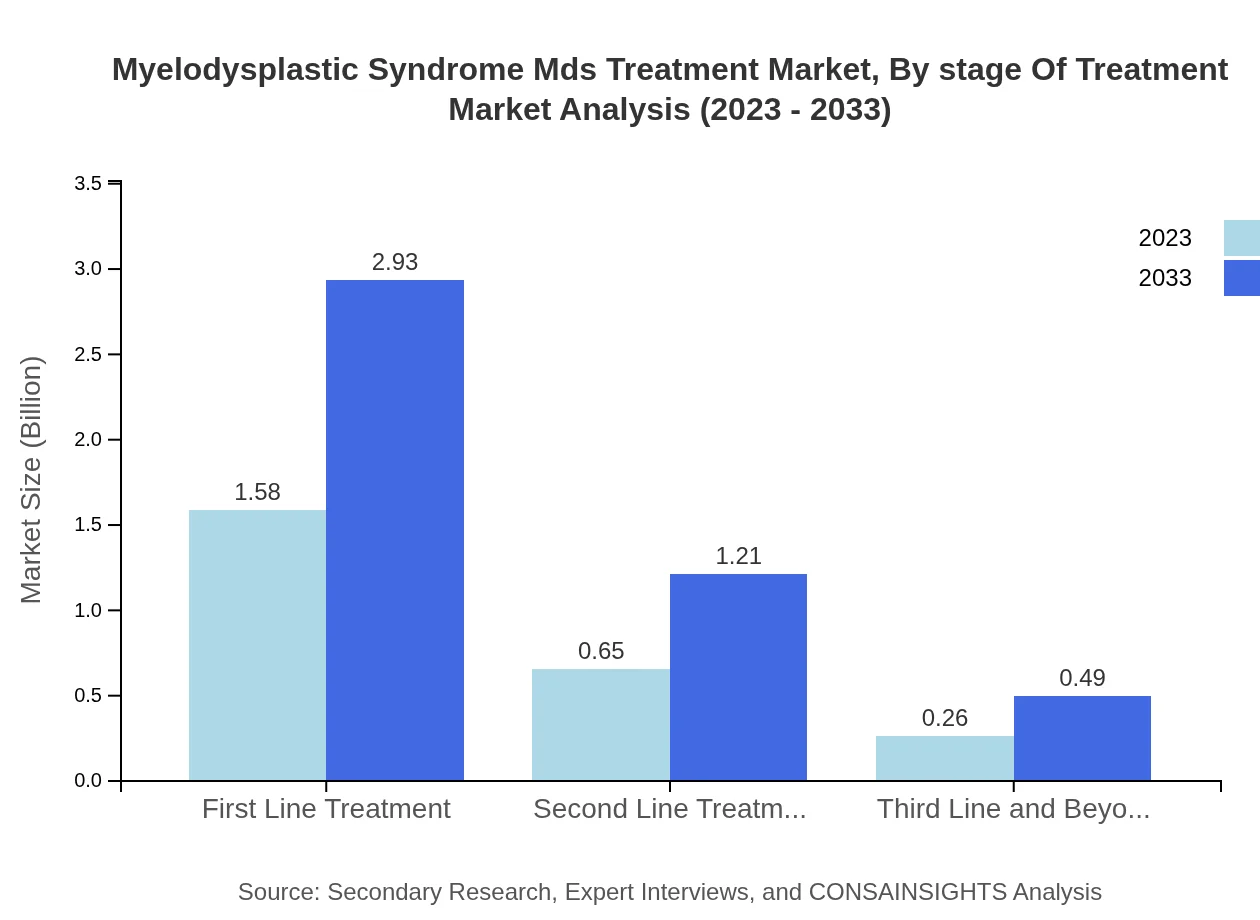
Myelodysplastic Syndrome Mds Treatment Market Analysis By Stage Of Treatment
The treatment market's segmentation by stage indicates that initial treatments capture the majority of market share, with first-line options valued at $1.58 billion and projected to increase to $2.93 billion. Subsequent stages, including second-line treatments grow moderately, reflecting a comprehensive care approach.
Myelodysplastic Syndrome Mds Treatment Market Trends and Future Forecast
Tell us your focus area and get a customized research report.
Global Market Leaders and Top Companies in Myelodysplastic Syndrome Mds Treatment Industry
Celgene Corporation:
Celgene is known for its breakthrough therapies for MDS, providing innovative solutions that improve patient outcomes through targeted treatment.Amgen Inc.:
Amgen specializes in biotechnology, focusing on novel approaches for treating hematologic diseases, including MDS.Novartis AG:
Novartis is heavily invested in research and development for innovative MDS treatments, including gene therapy approaches.Bristol-Myers Squibb:
Bristol-Myers Squibb develops advanced therapies for high-risk MDS patients, significantly impacting survival rates.Roche Holding AG:
Roche is recognized for its commitment to personalized medicine and has a portfolio for effective MDS management.We're grateful to work with incredible clients.









FAQs
What is the market size of myelodysplastic Syndrome Mds Treatment?
The market size for myelodysplastic syndrome (MDS) treatment is projected to reach $2.5 billion by 2033, growing at a CAGR of 6.2% from 2023. The growth is driven by increasing prevalence and advancements in treatment options.
What are the key market players or companies in the myelodysplastic Syndrome Mds Treatment industry?
Key players in the MDS treatment market include pharmaceutical giants like Novartis, Amgen, and Celgene, focusing on innovative drug development, clinical trials, and expanding product portfolios to meet increasing market demands.
What are the primary factors driving the growth in the myelodysplastic Syndrome Mds Treatment industry?
The growth drivers include rising incidences of MDS, increased awareness about blood disorders, advancements in therapeutic options, and significant investments in research and development for effective treatments.
Which region is the fastest Growing in the myelodysplastic Syndrome Mds Treatment?
The fastest-growing region in the MDS treatment market is North America, with projections indicating a market size increase from $0.85 billion in 2023 to $1.58 billion by 2033, driven by robust healthcare infrastructure.
Does ConsaInsights provide customized market report data for the myelodysplastic Syndrome Mds Treatment industry?
Yes, ConsaInsights offers customized market report data tailored to specific needs in the MDS treatment industry, ensuring relevant insights and data that align with unique business requirements.
What deliverables can I expect from this myelodysplastic Syndrome Mds Treatment market research project?
Expect comprehensive deliverables including in-depth market analysis, growth forecasts, competitive landscape assessments, regional insights, and trends specifically relevant to MDS treatment.
What are the market trends of myelodysplastic Syndrome Mds Treatment?
Current market trends include increasing utilization of targeted therapies, advancements in genomic medicine, and a shift towards personalized treatment plans, improving patient outcomes significantly.