Nanotechnology In Medical Devices Market Report
Published Date: 31 January 2026 | Report Code: nanotechnology-in-medical-devices
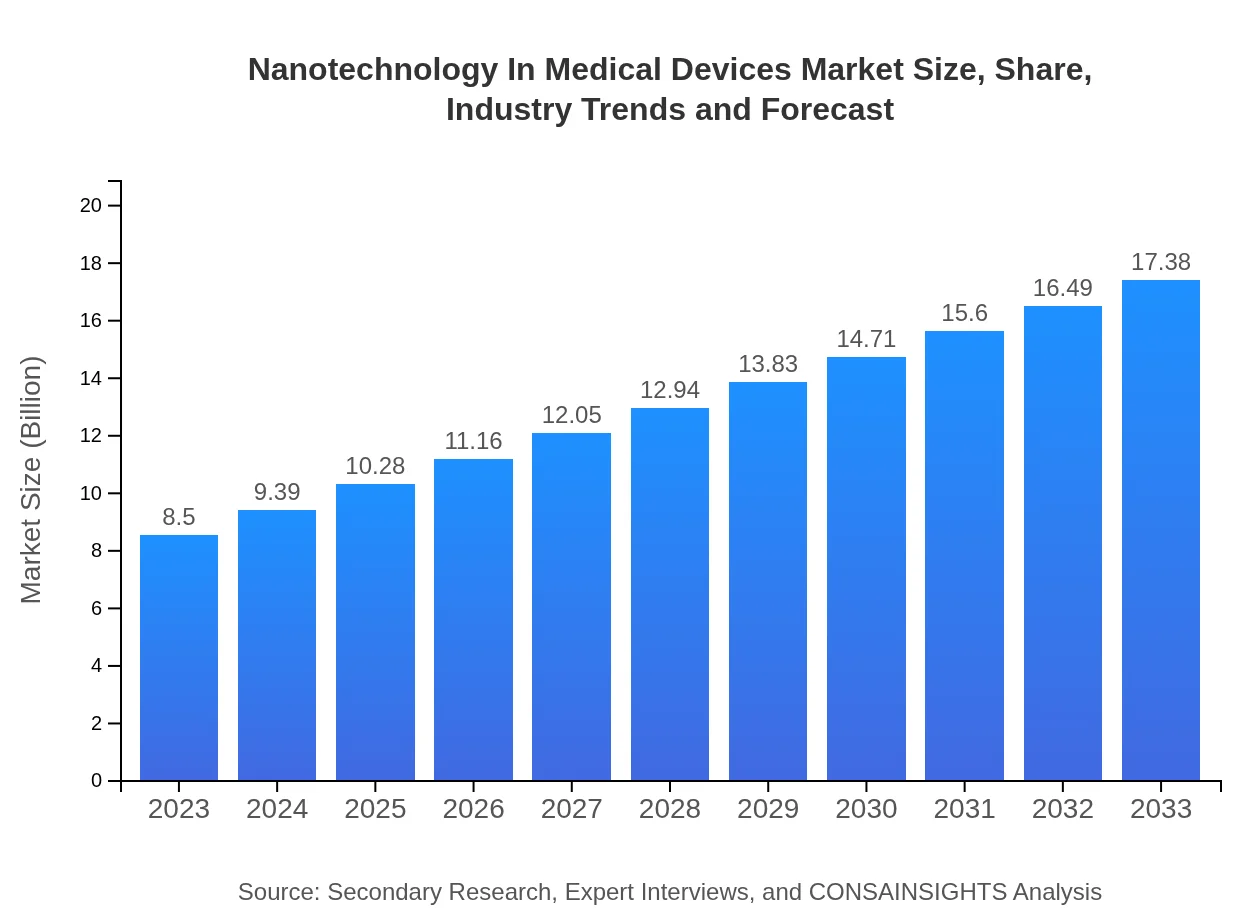
Nanotechnology In Medical Devices Market Size, Share, Industry Trends and Forecast to 2033
This report covers the Nanotechnology In Medical Devices market, providing insights into market size, growth projections, trends, and a regional analysis from 2023 to 2033.
| Metric | Value |
|---|---|
| Study Period | 2023 - 2033 |
| 2023 Market Size | $8.50 Billion |
| CAGR (2023-2033) | 7.2% |
| 2033 Market Size | $17.38 Billion |
| Top Companies | Johnson & Johnson, Medtronic , Thermo Fisher Scientific, Stryker Corporation, Siemens Healthineers |
| Last Modified Date | 31 January 2026 |
Nanotechnology In Medical Devices Market Overview
Customize Nanotechnology In Medical Devices Market Report market research report
- ✔ Get in-depth analysis of Nanotechnology In Medical Devices market size, growth, and forecasts.
- ✔ Understand Nanotechnology In Medical Devices's regional dynamics and industry-specific trends.
- ✔ Identify potential applications, end-user demand, and growth segments in Nanotechnology In Medical Devices
What is the Market Size & CAGR of Nanotechnology In Medical Devices market in 2023?
Nanotechnology In Medical Devices Industry Analysis
Nanotechnology In Medical Devices Market Segmentation and Scope
Tell us your focus area and get a customized research report.
Nanotechnology In Medical Devices Market Analysis Report by Region
Europe Nanotechnology In Medical Devices Market Report:
Europe is anticipated to see growth from USD 2.94 billion in 2023 to USD 6.01 billion in 2033, indicating a CAGR of 7.4%. The European market is expanding due to strong regulatory frameworks and a push for innovative medical solutions.Asia Pacific Nanotechnology In Medical Devices Market Report:
In the Asia Pacific region, the Nanotechnology In Medical Devices market is projected to grow from USD 1.56 billion in 2023 to USD 3.19 billion in 2033, reflecting a compound annual growth rate (CAGR) of 8.6%. The region benefits from substantial investments in healthcare infrastructure and a growing emphasis on advanced diagnostic and therapeutic modalities.North America Nanotechnology In Medical Devices Market Report:
North America leads the market with a robust valuation, expected to rise from USD 2.84 billion in 2023 to USD 5.80 billion in 2033, boasting a significant CAGR of 7.6%. The region is home to several leading biomedical companies and research institutions focused on nanotechnology innovations.South America Nanotechnology In Medical Devices Market Report:
The South American market is smaller, with values increasing from USD 0.14 billion in 2023 to USD 0.29 billion in 2033 at a CAGR of 7.6%. Market growth is driven by rising healthcare expenditures and the gradual adoption of advanced medical technologies.Middle East & Africa Nanotechnology In Medical Devices Market Report:
The Middle East and Africa market is improving, increasing from USD 1.02 billion in 2023 to USD 2.09 billion in 2033. With a CAGR of 7.8%, the region's healthcare investments are enhancing the adoption of nanotechnology in medical devices.Tell us your focus area and get a customized research report.
Nanotechnology In Medical Devices Market Analysis Nanotechnology_in_medical_devices_market_by_product
Global Nanotechnology in Medical Devices Market, By Product Market Analysis (2023 - 2033)
The product segments in this market include drug delivery systems, diagnostic devices, implants, and nanomaterials. Drug delivery systems, leading the market, are expected to grow significantly with advances in targeting methods, while diagnostic devices are gaining traction due to improved accuracy and efficacy.
Nanotechnology In Medical Devices Market Analysis Nanotechnology_in_medical_devices_market_by_application
Global Nanotechnology in Medical Devices Market, By Application Market Analysis (2023 - 2033)
Applications of nanotechnology span from advanced imaging systems to therapies aimed at precision medicine. The application segments highlight the growing inclination towards personalized healthcare solutions assisted by nanotechnology.
Nanotechnology In Medical Devices Market Analysis Nanotechnology_in_medical_devices_market_by_end_user
Global Nanotechnology in Medical Devices Market, By End-User Market Analysis (2023 - 2033)
End-user segments consist of hospitals, research institutes, and diagnostic laboratories. Hospitals lead in terms of market share and have increasingly integrated these technologies into their operations to enhance patient outcomes.
Nanotechnology In Medical Devices Market Analysis Nanotechnology_in_medical_devices_market_by_technological_advancements
Global Nanotechnology in Medical Devices Market, By Technological Advancements Market Analysis (2023 - 2033)
This segment focuses on advancements in nanoparticle formulations, nanobiosensors, and nanoimaging techniques. Research and development efforts are crucial to delivering innovative solutions that address current medical challenges.
Nanotechnology In Medical Devices Market Analysis Nanotechnology_in_medical_devices_market_by_regulatory_environment
Global Nanotechnology in Medical Devices Market, By Regulatory Environment Market Analysis (2023 - 2033)
The role of regulatory frameworks is significant in ensuring the safety and efficacy of nanotechnology applications in medical devices. Ongoing collaborations between industry leaders and regulatory bodies are essential to streamline approvals and ensure public health standards.
Nanotechnology In Medical Devices Market Trends and Future Forecast
Tell us your focus area and get a customized research report.
Global Market Leaders and Top Companies in Nanotechnology In Medical Devices Industry
Johnson & Johnson:
A major player in the healthcare space, Johnson & Johnson has incorporated nanotechnology to develop advanced drug delivery systems and innovative implant designs.Medtronic :
Medtronic is recognized for its contributions to the field of medical devices and has pioneered the use of nanotechnology in various therapeutic and diagnostic solutions.Thermo Fisher Scientific:
Known for its cutting-edge laboratory equipment, Thermo Fisher integrates nanotechnology into biotechnology and pharmaceutical solutions.Stryker Corporation:
Specializing in surgical equipment and devices, Stryker is leveraging nanotechnology for better implant and surgical solutions.Siemens Healthineers:
Siemens is enhancing imaging diagnostics through the application of nanotechnology, providing high-resolution imaging products.We're grateful to work with incredible clients.









FAQs
What is the market size of nanotechnology in medical devices?
The market size of nanotechnology in medical devices is projected to reach approximately $8.5 billion by 2033, growing at a CAGR of 7.2% from its current valuation. This growth is driven by the increasing demand for advanced medical technologies.
What are the key market players or companies in this industry?
Key players in the nanotechnology in medical devices industry include major companies such as Medtronic, Abbott Laboratories, and Siemens Healthineers, among others. These companies focus on innovative solutions that leverage nanotechnology to enhance medical devices.
What are the primary factors driving the growth in the nanotechnology in medical devices industry?
The growth in the nanotechnology in medical devices industry is primarily driven by advancements in technology, increasing investments in healthcare R&D, and a rising prevalence of chronic diseases that necessitate the development of innovative medical solutions.
Which region is the fastest Growing in the nanotechnology in medical devices market?
The fastest-growing region in the nanotechnology in medical devices market is Europe, which is expected to grow from $2.94 billion in 2023 to $6.01 billion by 2033, reflecting a strong demand for advanced medical technologies.
Does ConsInsights provide customized market report data for the nanotechnology in medical devices industry?
Yes, ConsInsights offers customizable market report data tailored to specific needs in the nanotechnology in medical devices industry, enabling clients to access detailed insights relevant to their unique market challenges.
What deliverables can I expect from this nanotechnology in medical devices market research project?
Deliverables from this market research project include comprehensive market analysis, future growth forecasts, competitive landscape evaluation, and segmentation insights, providing a thorough understanding of industry dynamics and opportunities.
What are the market trends of nanotechnology in medical devices?
Current market trends in nanotechnology in medical devices include the increasing integration of nanotechnology in drug delivery systems and diagnostic devices, alongside a growing focus on personalized medicine and the development of smart medical devices.