Needle Free Drug Delivery Devices Market Report
Published Date: 31 January 2026 | Report Code: needle-free-drug-delivery-devices
Needle Free Drug Delivery Devices Market Size, Share, Industry Trends and Forecast to 2033
This report provides a comprehensive analysis of the Needle Free Drug Delivery Devices market, detailing current market conditions, segmentation, regional insights, industry trends, and forecasts from 2023 to 2033.
| Metric | Value |
|---|---|
| Study Period | 2023 - 2033 |
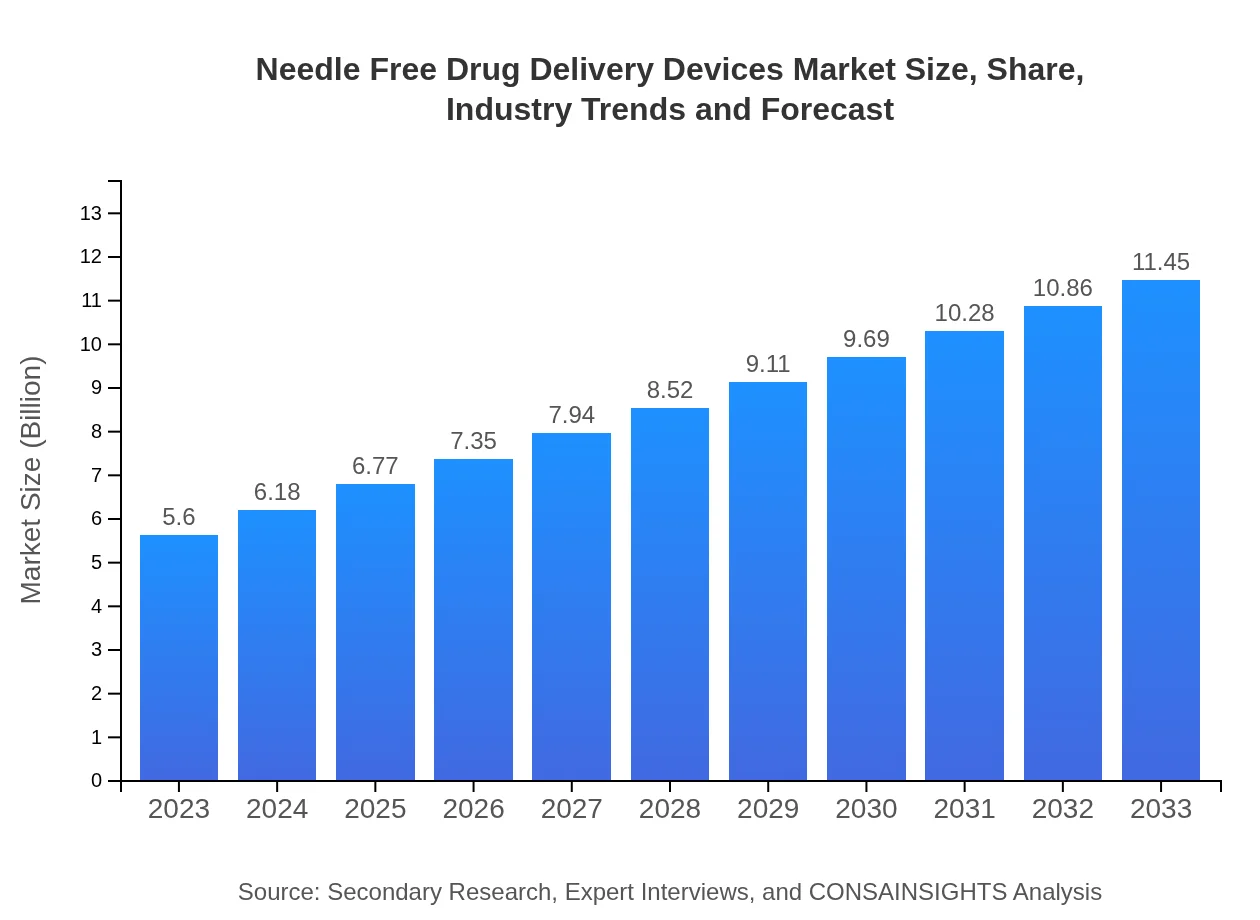
| 2023 Market Size | $5.60 Billion |
| CAGR (2023-2033) | 7.2% |
| 2033 Market Size | $11.45 Billion |
| Top Companies | BD Medical, PharmaJet, Zogenix, Syringe Technology |
| Last Modified Date | 31 January 2026 |
Needle Free Drug Delivery Devices Market Overview
Customize Needle Free Drug Delivery Devices Market Report market research report
- ✔ Get in-depth analysis of Needle Free Drug Delivery Devices market size, growth, and forecasts.
- ✔ Understand Needle Free Drug Delivery Devices's regional dynamics and industry-specific trends.
- ✔ Identify potential applications, end-user demand, and growth segments in Needle Free Drug Delivery Devices
What is the Market Size & CAGR of Needle Free Drug Delivery Devices market in 2023 and 2033?
Needle Free Drug Delivery Devices Industry Analysis
Needle Free Drug Delivery Devices Market Segmentation and Scope
Tell us your focus area and get a customized research report.
Needle Free Drug Delivery Devices Market Analysis Report by Region
Europe Needle Free Drug Delivery Devices Market Report:
In Europe, the market size is projected to grow from USD 1.60 billion in 2023 to USD 3.27 billion by 2033, driven by strict regulations advocating for patient safety and a growing elderly population requiring medication.Asia Pacific Needle Free Drug Delivery Devices Market Report:
As of 2023, the Asia Pacific market size is valued at USD 1.06 billion and is expected to reach USD 2.16 billion by 2033, witnessing substantial growth driven by rising healthcare infrastructure and increasing prevalence of chronic diseases.North America Needle Free Drug Delivery Devices Market Report:
The North American market, valued at USD 2.10 billion in 2023, is forecasted to nearly double by 2033, reaching an estimated USD 4.30 billion. The growth expectation arises from technological innovations and the high prevalence of chronic conditions requiring regular medication.South America Needle Free Drug Delivery Devices Market Report:
Currently valued at USD 0.47 billion in 2023, the South American Needle Free Drug Delivery Devices market is projected to grow to USD 0.95 billion by 2033, driven by increasing vaccination campaigns and awareness regarding needle-free technologies.Middle East & Africa Needle Free Drug Delivery Devices Market Report:
The market in the Middle East and Africa, currently valued at USD 0.37 billion, is estimated to grow to USD 0.76 billion by 2033, influenced by improving healthcare systems and rising obesity rates.Tell us your focus area and get a customized research report.
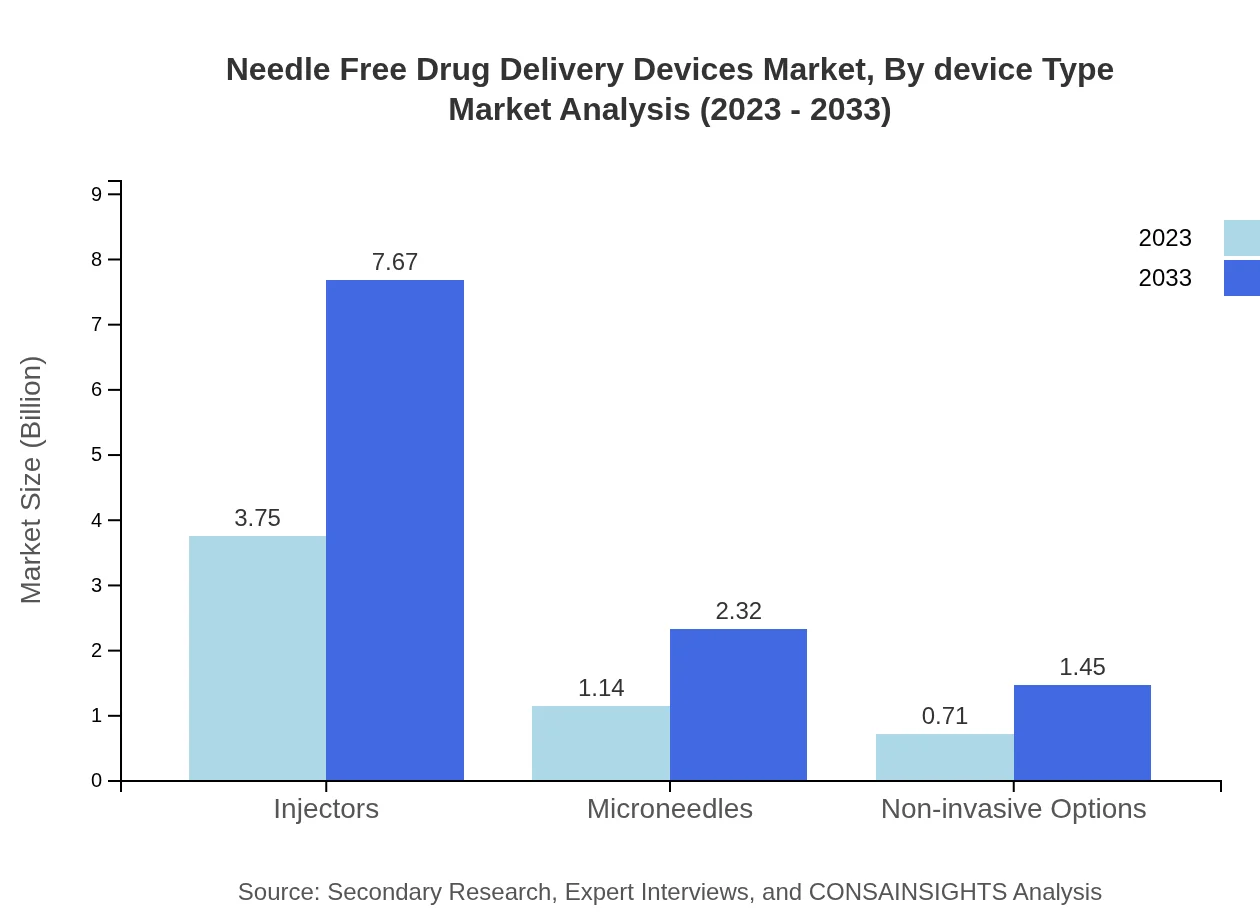
Needle Free Drug Delivery Devices Market Analysis By Device Type
The Needle-free Drug Delivery Devices market by device type consists predominantly of Jet Injectors and Microneedle technologies. While Jet Injectors accounted for a substantial market share due to their efficiency in delivering vaccines and medications without needles, Microneedles are gaining traction for their versatility in drug delivery applications. In 2023, the market size for Injectors is around USD 3.75 billion, rising to USD 7.67 billion by 2033, reflecting their dominance in the market.
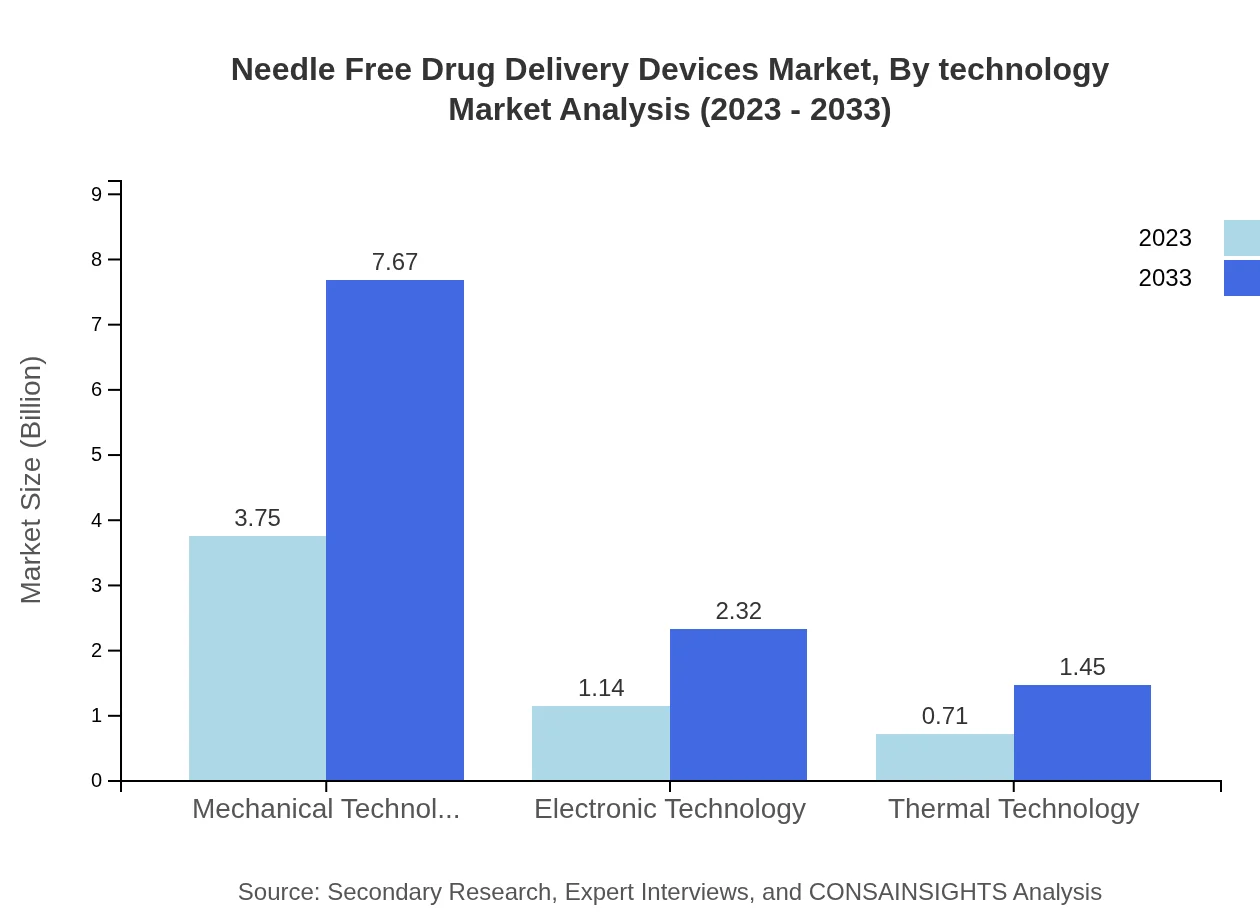
Needle Free Drug Delivery Devices Market Analysis By Technology
The market segments by technology include Mechanical Technology, Electronic Technology, and Thermal Technology. Mechanical technology remains the most dominant segment, accounting for over 67% of the market share in 2023, with an increase to 67.03% by 2033. Electronic technology follows with a significant market presence, while Thermal technology shows increasing relevance in specialized applications.
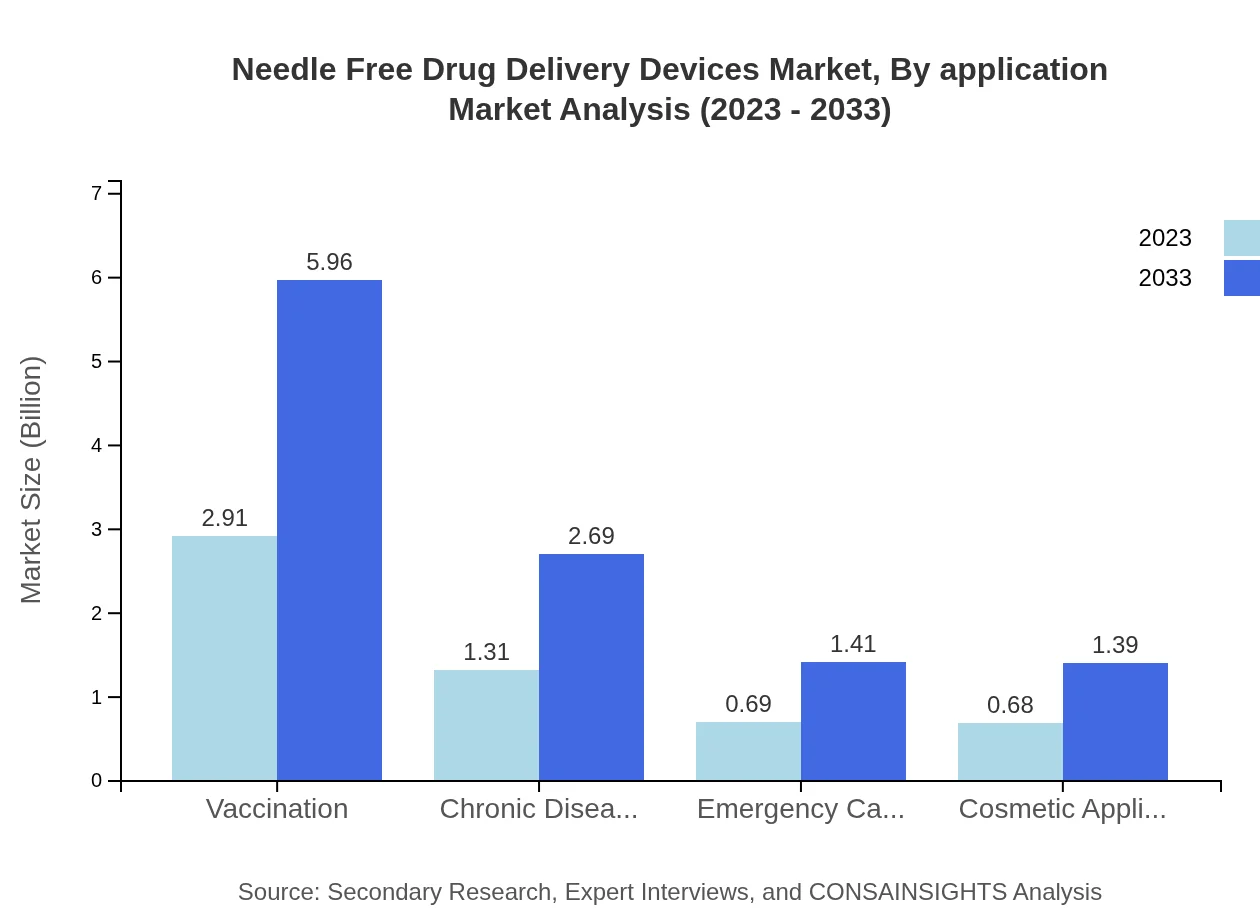
Needle Free Drug Delivery Devices Market Analysis By Application
Segmenting by application reveals significant categories such as Vaccination, Chronic Diseases Management, Emergency Care, and Cosmetic Applications. Vaccination continues to be the leading application area, accounting for 52% in 2023, with projections of similar growth due to ongoing vaccination drives globally. Chronic Diseases Management shows increasing demand given the rising prevalence of diabetes and other chronic conditions.
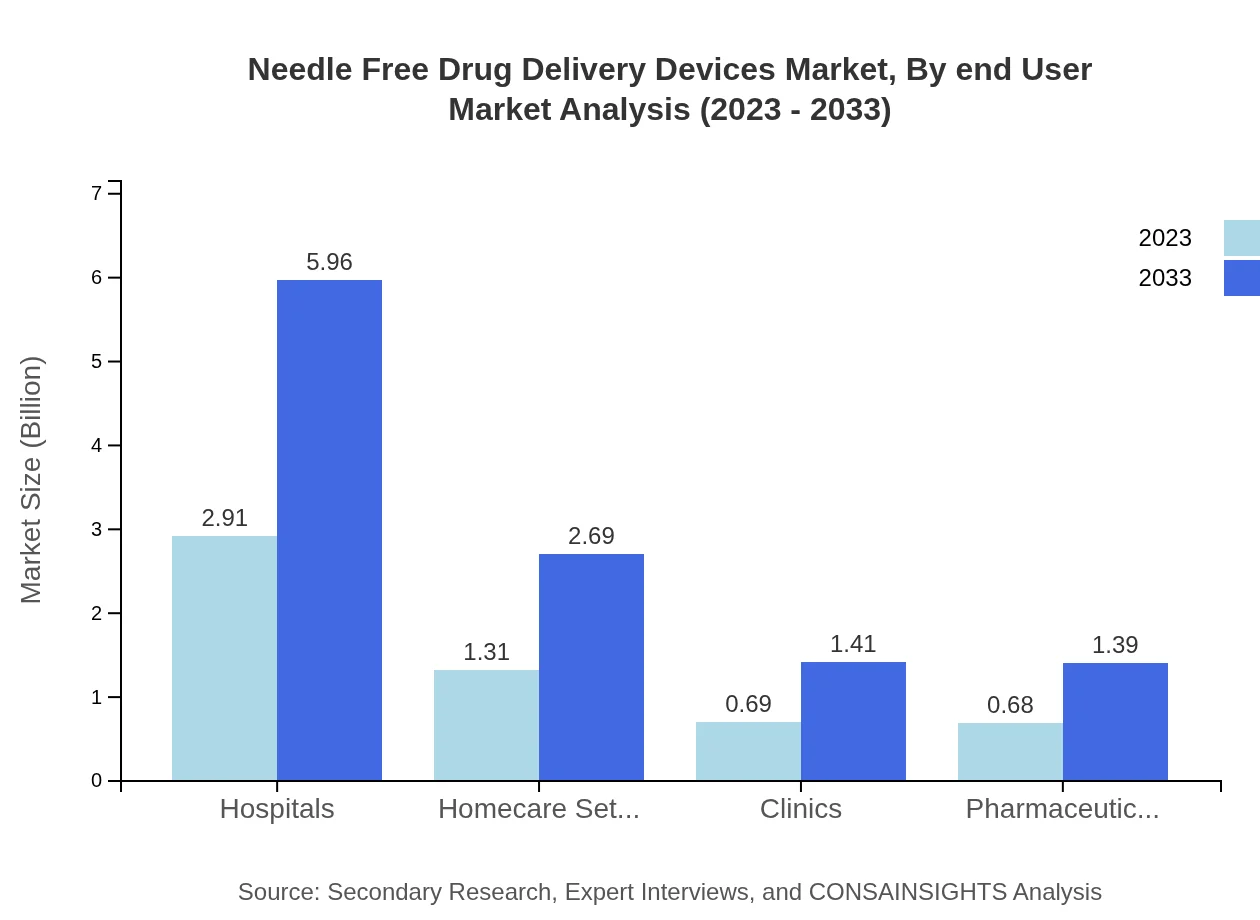
Needle Free Drug Delivery Devices Market Analysis By End User
Market segmentation by end-users encompasses Hospitals, Homecare Settings, Clinics, and Pharmaceutical Companies. Hospitals account for the largest market share at 52.03% in 2023, which is projected to remain stable over the forecast period, reflecting the sustained demand for advanced drug delivery methods within healthcare facilities.
Needle Free Drug Delivery Devices Market Trends and Future Forecast
Tell us your focus area and get a customized research report.
Global Market Leaders and Top Companies in Needle Free Drug Delivery Devices Industry
BD Medical:
A leading company specializing in injection devices, BD Medical offers advanced needle-free injection solutions focusing on safety and efficiency.PharmaJet:
PharmaJet is a pioneer in the field of needle-free injection technology, delivering innovative solutions aimed at improving vaccine delivery and patient compliance.Zogenix:
Zogenix develops and commercializes needle-free injection devices intended for various health applications, enhancing drug delivery systems globally.Syringe Technology:
Specializes in developing advanced needle-free injection technologies that ensure patient safety and comfort across numerous therapeutic applications.We're grateful to work with incredible clients.









FAQs
What is the market size of Needle-Free Drug Delivery Devices?
The Needle-Free Drug Delivery Devices market was valued at approximately $5.6 billion in 2023, with a projected growth rate of 7.2% CAGR through 2033, indicating significant expansion opportunities.
What are the key market players or companies in the Needle-Free Drug Delivery Devices industry?
Key players in the Needle-Free Drug Delivery Devices market include Medtronic, BD, and Zephyr Technik among others. These companies are important contributors to the sector, driving advancements in technology and market expansion.
What are the primary factors driving the growth in the Needle-Free Drug Delivery Devices industry?
Growth in the Needle-Free Drug Delivery Devices sector is primarily driven by increasing demand for painless drug delivery solutions, a rise in chronic disease prevalence, and advancements in technology that enhance device efficiency.
Which region is the fastest Growing in the Needle-Free Drug Delivery Devices market?
Asia-Pacific is the fastest-growing region in the Needle-Free Drug Delivery Devices market, projected to grow from $1.06 billion in 2023 to $2.16 billion by 2033, emphasizing the region's expanding healthcare infrastructure.
Does ConsaInsights provide customized market report data for the Needle-Free Drug Delivery Devices industry?
Yes, ConsaInsights offers customized market report data tailored to specific needs within the Needle-Free Drug Delivery Devices industry, ensuring clients receive relevant insights and analysis.
What deliverables can I expect from this Needle-Free Drug Delivery Devices market research project?
Deliverables from the Needle-Free Drug Delivery Devices market research project include comprehensive reports, segmentation analysis, regional insights, market trends, competitive landscape, and strategic recommendations.
What are the market trends of Needle-Free Drug Delivery Devices?
Market trends for Needle-Free Drug Delivery Devices include increasing usage in homecare settings, rising adoption of electronic and mechanical technologies, and a shift towards non-invasive delivery methods across various medical applications.