Neonatal And Prenatal Devices Market Report
Published Date: 31 January 2026 | Report Code: neonatal-and-prenatal-devices
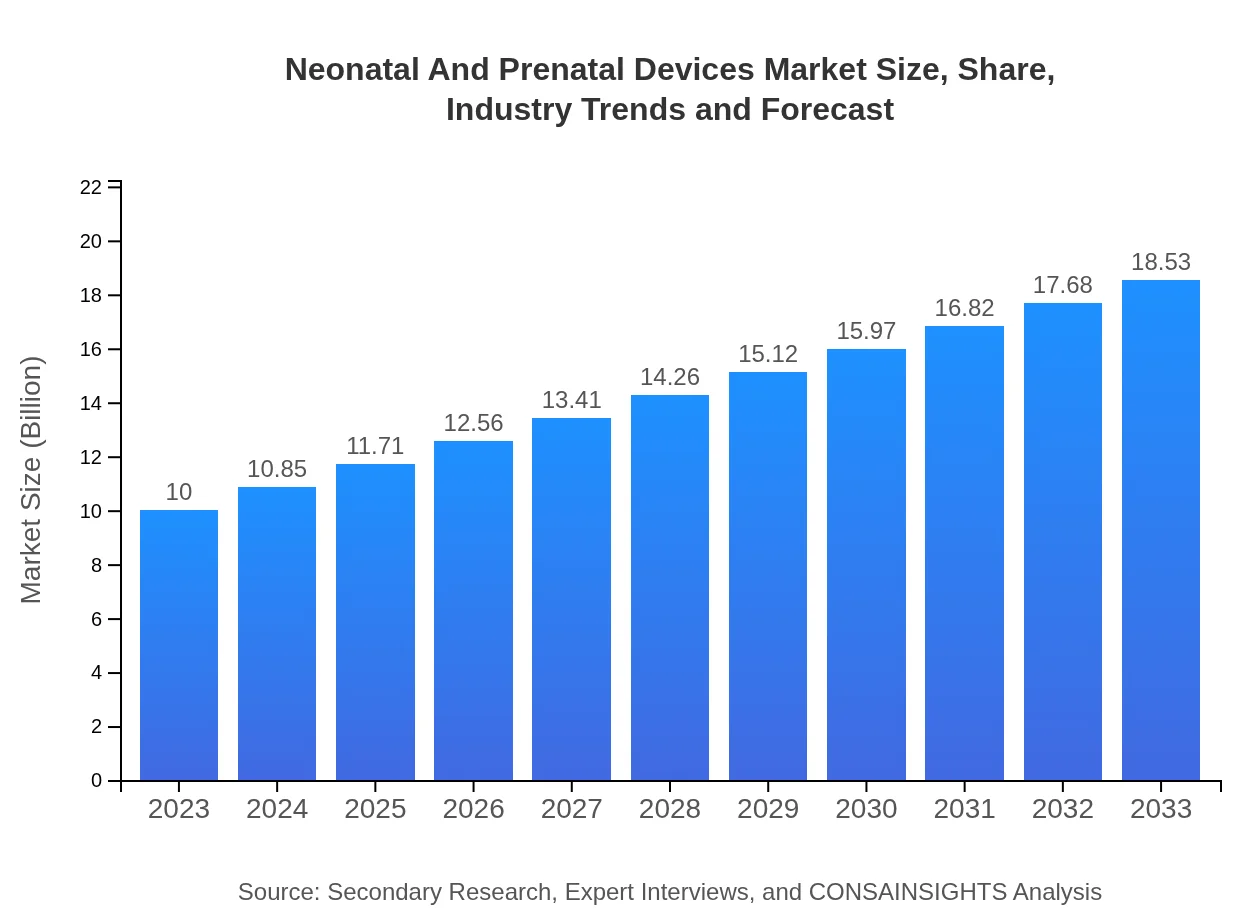
Neonatal And Prenatal Devices Market Size, Share, Industry Trends and Forecast to 2033
This report delves into the Neonatal and Prenatal Devices market, providing comprehensive insights including market size, growth forecasts from 2023 to 2033, regional analysis, industry trends, and competitive landscape.
| Metric | Value |
|---|---|
| Study Period | 2023 - 2033 |
| 2023 Market Size | $10.00 Billion |
| CAGR (2023-2033) | 6.2% |
| 2033 Market Size | $18.53 Billion |
| Top Companies | General Electric Company, Philips Healthcare, Siemens Healthineers, Medtronic , Draegerwerk AG |
| Last Modified Date | 31 January 2026 |
Neonatal And Prenatal Devices Market Overview
Customize Neonatal And Prenatal Devices Market Report market research report
- ✔ Get in-depth analysis of Neonatal And Prenatal Devices market size, growth, and forecasts.
- ✔ Understand Neonatal And Prenatal Devices's regional dynamics and industry-specific trends.
- ✔ Identify potential applications, end-user demand, and growth segments in Neonatal And Prenatal Devices
What is the Market Size & CAGR of Neonatal And Prenatal Devices market in 2023?
Neonatal And Prenatal Devices Industry Analysis
Neonatal And Prenatal Devices Market Segmentation and Scope
Tell us your focus area and get a customized research report.
Neonatal And Prenatal Devices Market Analysis Report by Region
Europe Neonatal And Prenatal Devices Market Report:
Europe's market is set to grow from $2.70 billion in 2023 to $5.00 billion by 2033, driven by increasing government initiatives and the presence of key players. However, the region faces challenges related to stringent regulations and reimbursement issues.Asia Pacific Neonatal And Prenatal Devices Market Report:
In the Asia Pacific region, the Neonatal and Prenatal Devices market was valued at $2.14 billion in 2023, projected to reach $3.96 billion by 2033. The growth is driven by increasing healthcare investments and rising birth rates, although variations in healthcare access remain a concern.North America Neonatal And Prenatal Devices Market Report:
North America leads the market, valued at approximately $3.37 billion in 2023, with forecasts of $6.24 billion by 2033. The region benefits from advanced healthcare systems, high adoption of new technologies, and a robust regulatory environment that fosters innovation.South America Neonatal And Prenatal Devices Market Report:
The South American market is comparatively smaller, valued at $0.58 billion in 2023, with expectations of reaching $1.07 billion by 2033. Economic factors and healthcare challenges may hinder faster growth, but increasing awareness is paving the way for better prenatal care.Middle East & Africa Neonatal And Prenatal Devices Market Report:
The Middle East and Africa market has a value of $1.22 billion in 2023, projected to grow to $2.26 billion by 2033. Growth in this region is hampered by economic instability and increased healthcare constraints, yet substantial opportunities still exist, especially within urban areas.Tell us your focus area and get a customized research report.
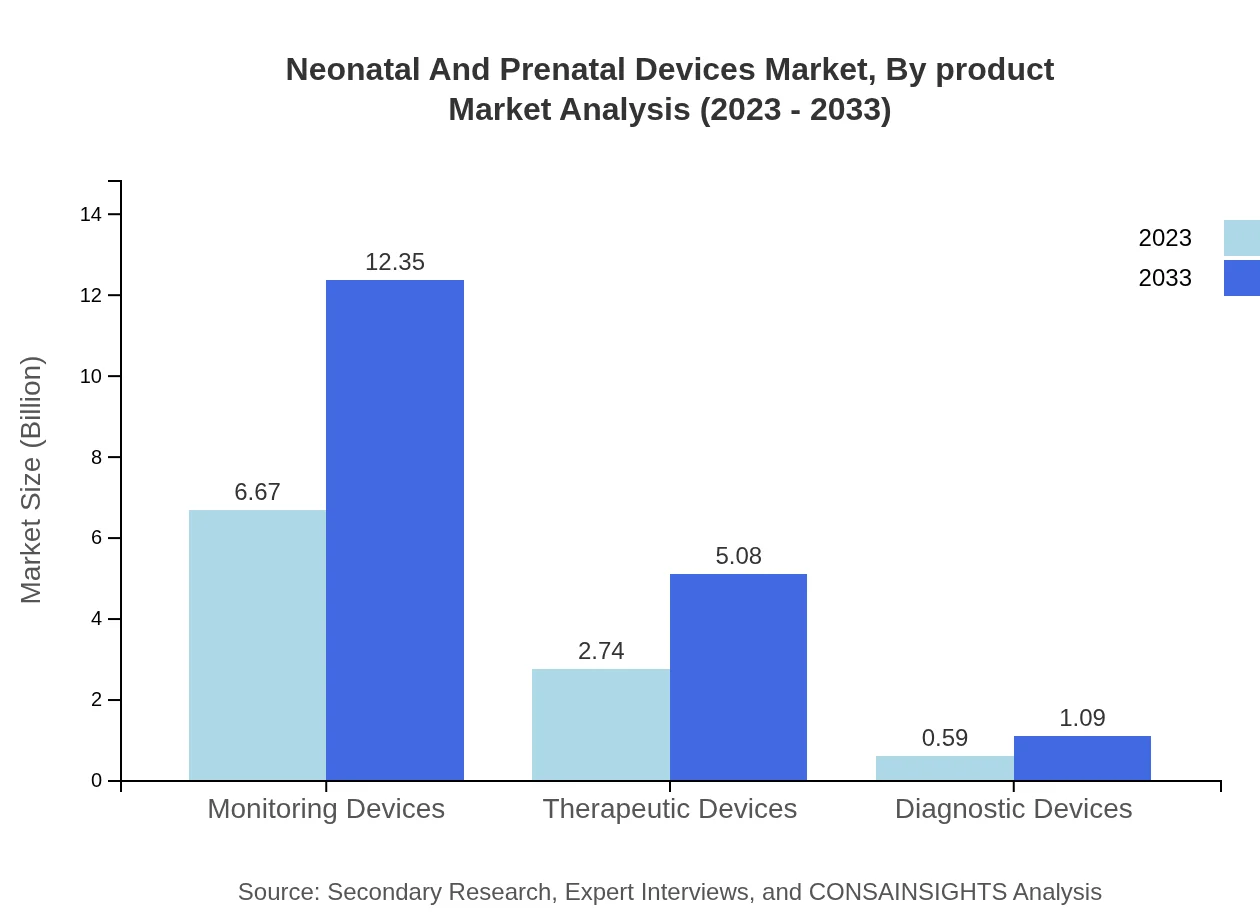
Neonatal And Prenatal Devices Market Analysis By Product
Monitoring devices constitute the largest market share, valued at $6.67 billion in 2023, and projected to reach $12.35 billion by 2033. Therapeutic devices follow, indicating a steady growth trend due to rising healthcare demand.
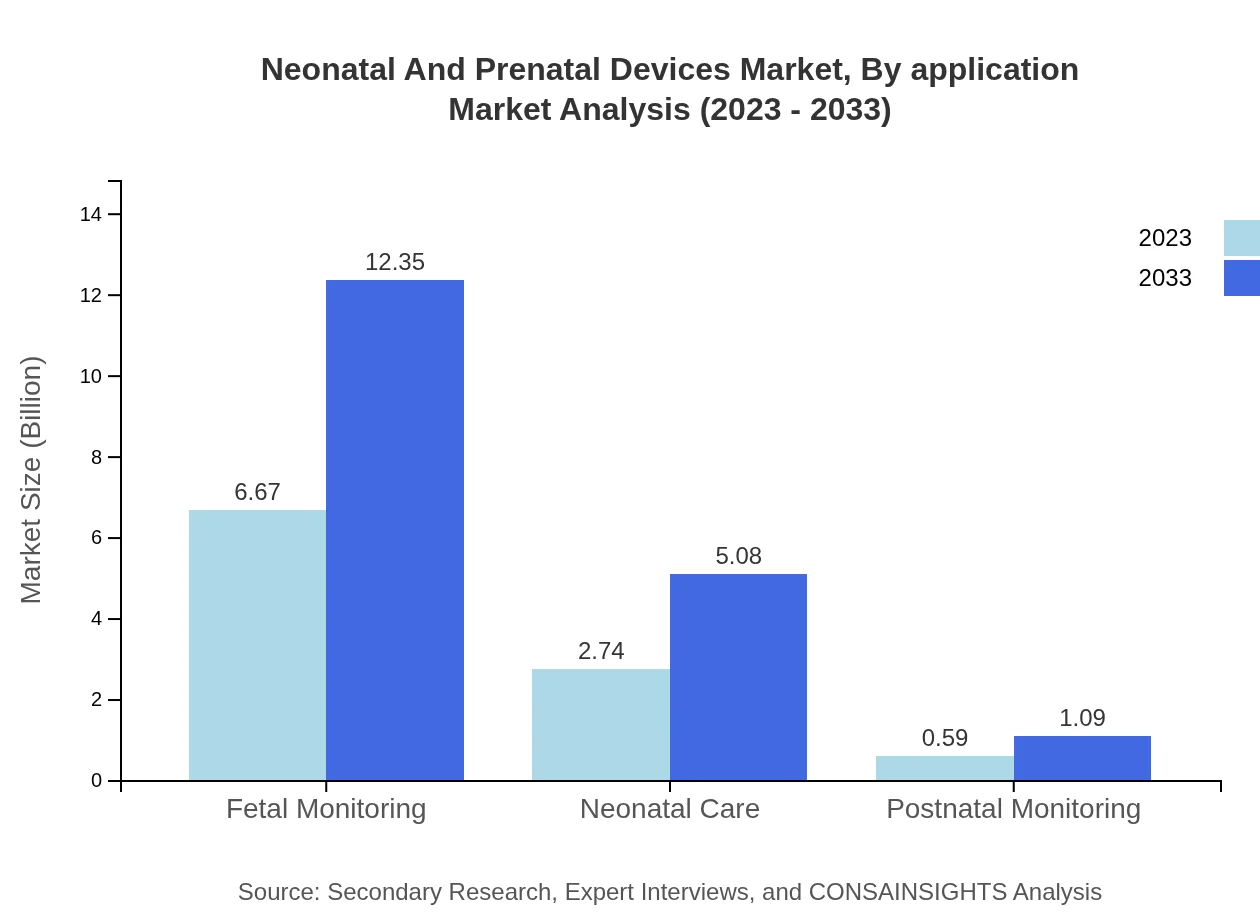
Neonatal And Prenatal Devices Market Analysis By Application
The market is segmented into applications such as fetal monitoring, neonatal care, and postnatal monitoring. The fetal monitoring segment, valued at $6.67 billion in 2023, shows consistent growth aligned with increased prenatal care awareness.
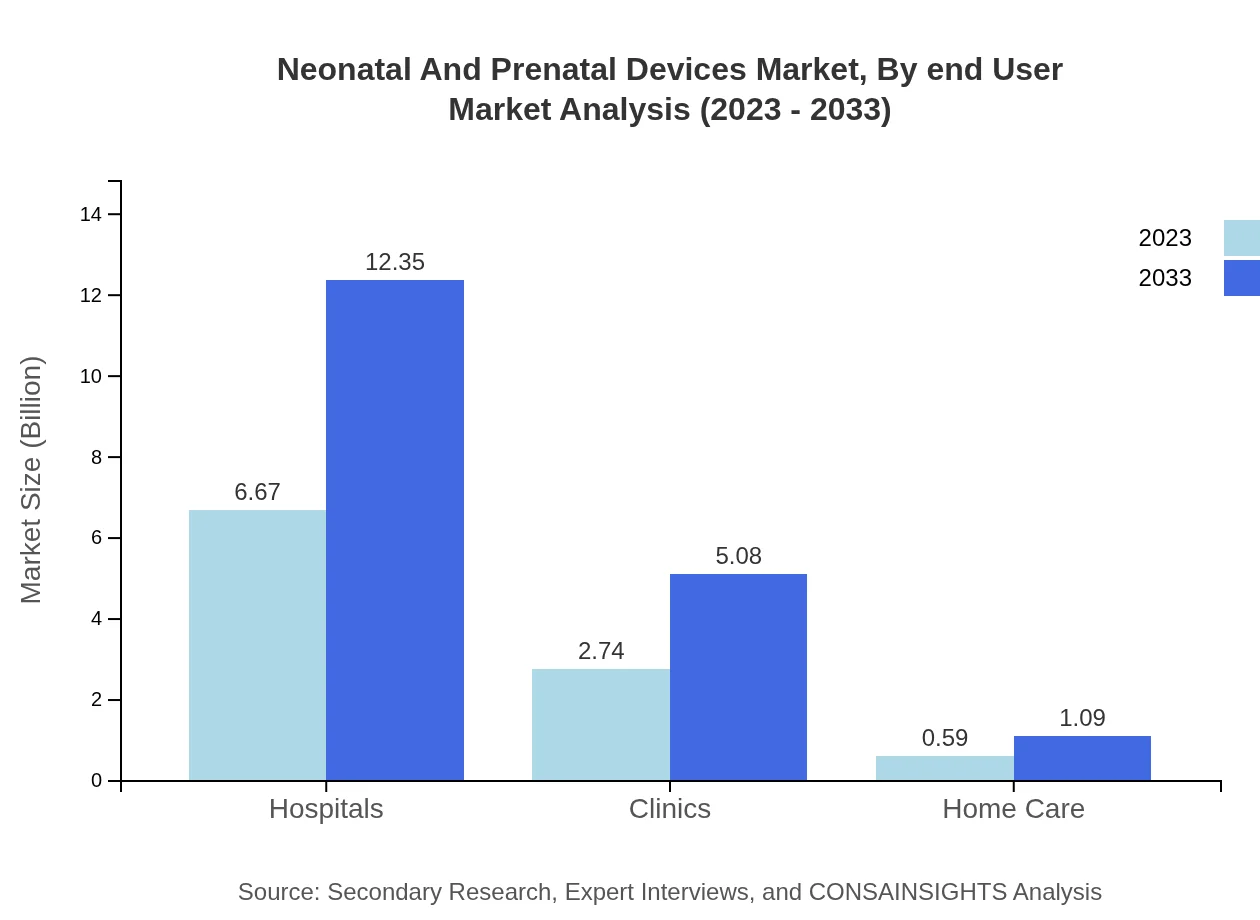
Neonatal And Prenatal Devices Market Analysis By End User
Hospitals dominate the end-user segment, capturing $6.67 billion in 2023. Clinics are also significant contributors, valued at $2.74 billion for the same year, highlighting the sector's reliance on professional healthcare settings.
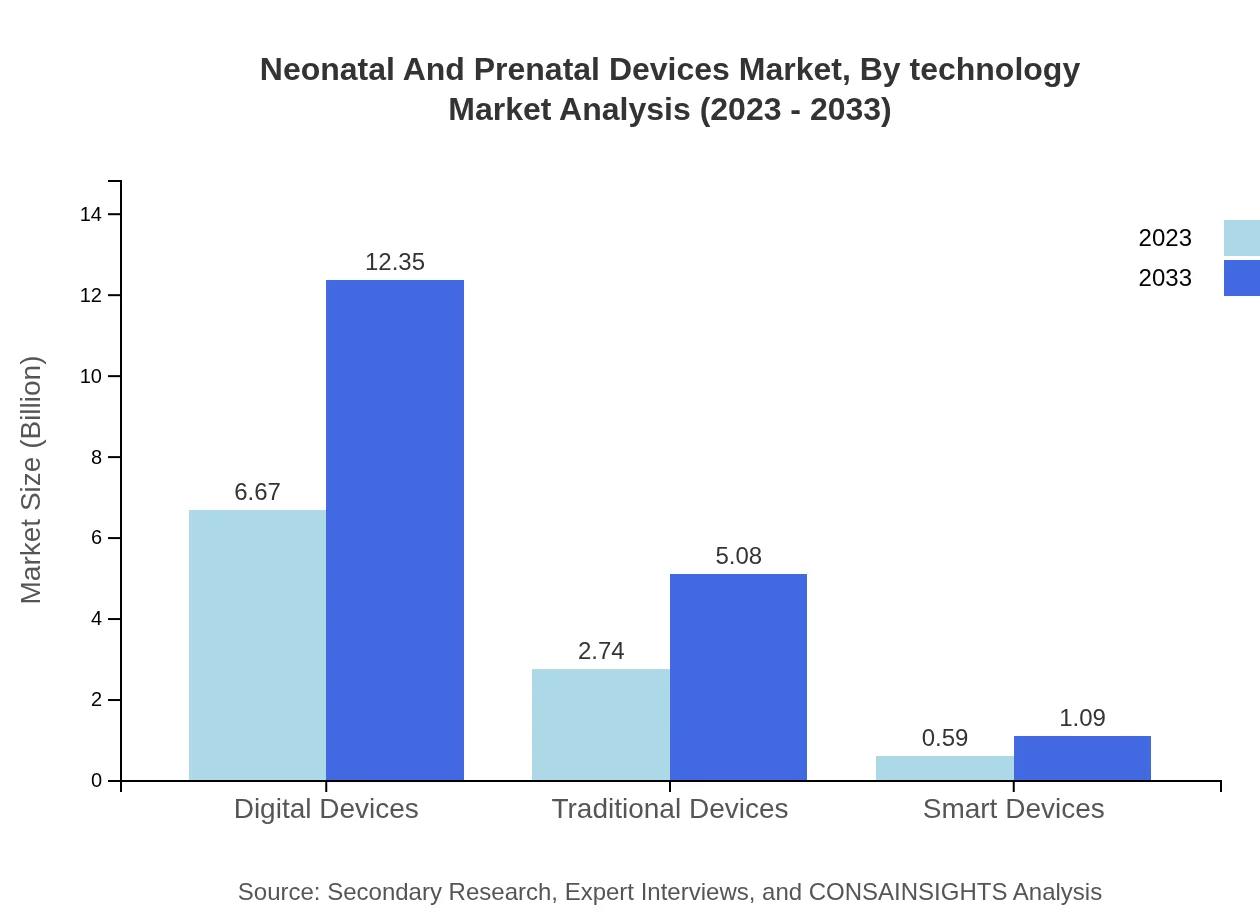
Neonatal And Prenatal Devices Market Analysis By Technology
Digital devices lead the technology segment with strong growth, valued at $6.67 billion in 2023. Traditional devices show stable performance, but smart devices are emerging, although they currently stand at $0.59 billion in 2023.
Neonatal And Prenatal Devices Market Analysis By End_user_segment
Global Neonatal and Prenatal Devices Market, By End-User Segment Market Analysis (2023 - 2033)
End-user segmentation indicates that hospitals are the primary users of neonatal and prenatal devices, followed by clinics. Home care setups are gradually gaining traction, especially with the rise of at-home monitoring solutions.
Neonatal And Prenatal Devices Market Trends and Future Forecast
Tell us your focus area and get a customized research report.
Global Market Leaders and Top Companies in Neonatal And Prenatal Devices Industry
General Electric Company:
GE Healthcare provides a broad range of neonatal and prenatal solutions, focusing on innovation and advanced technologies, actively contributing to improving patient care outcomes.Philips Healthcare:
Philips is a leader in medical devices, offering cutting-edge prenatal and neonatal monitoring solutions, innovating to enhance medical outcomes and patient experiences.Siemens Healthineers:
Siemens Healthineers specializes in medical devices, including neonatal care. Their commitment to technological advancements sets them apart in the industry.Medtronic :
Medtronic's comprehensive neonatal solutions focus on critical care, improving clinical efficiencies and patient outcomes through robust technology.Draegerwerk AG:
Draeger focuses on innovative technologies for neonatal devices, leveraging extensive research and market knowledge to provide high-quality care solutions.We're grateful to work with incredible clients.









FAQs
What is the market size of neonatal And Prenatal Devices?
The global neonatal and prenatal devices market is projected to be valued at approximately $10 billion in 2023, with a CAGR of 6.2% expected through 2033. This growth reflects increasing demand for advanced medical technologies in maternal and infant care.
What are the key market players or companies in the neonatal And Prenatal Devices industry?
While the specific players are not provided, the neonatal and prenatal devices industry typically includes major medical device manufacturers, healthcare companies, and specialized startups focusing on maternal and infant healthcare solutions.
What are the primary factors driving the growth in the neonatal And Prenatal Devices industry?
Primary growth drivers include increased awareness of maternal and infant health, technological advancements in monitoring devices, rising healthcare expenditure, and supportive government initiatives aimed at improving prenatal and neonatal care worldwide.
Which region is the fastest Growing in the neonatal And Prenatal Devices market?
The fastest-growing region for neonatal and prenatal devices is North America, expected to grow from $3.37 billion in 2023 to $6.24 billion by 2033. This growth is fueled by high healthcare spending and advanced medical infrastructure.
Does ConsaInsights provide customized market report data for the neonatal And Prenatal Devices industry?
Yes, ConsaInsights offers customized market report data tailored to specific client needs in the neonatal and prenatal devices industry, allowing for in-depth analysis and insights based on individual business requirements.
What deliverables can I expect from this neonatal And Prenatal Devices market research project?
Deliverables from the neonatal and prenatal devices market research project include comprehensive market analysis reports, segmentation data, competitive landscape assessments, forecasts, and actionable insights to support strategic decision-making.
What are the market trends of neonatal And Prenatal Devices?
Key market trends include an increasing shift towards digital and smart devices, growth in prenatal monitoring technologies, and a rising demand for home-based care solutions, reflecting evolving patient preferences in neonatal and prenatal care.