Nephrostomy Devices Market Report
Published Date: 31 January 2026 | Report Code: nephrostomy-devices
Nephrostomy Devices Market Size, Share, Industry Trends and Forecast to 2033
This report offers a comprehensive analysis of the nephrostomy devices market from 2023 to 2033, detailing market size, trends, regional insights, and key player analyses to help stakeholders make informed decisions.
| Metric | Value |
|---|---|
| Study Period | 2023 - 2033 |
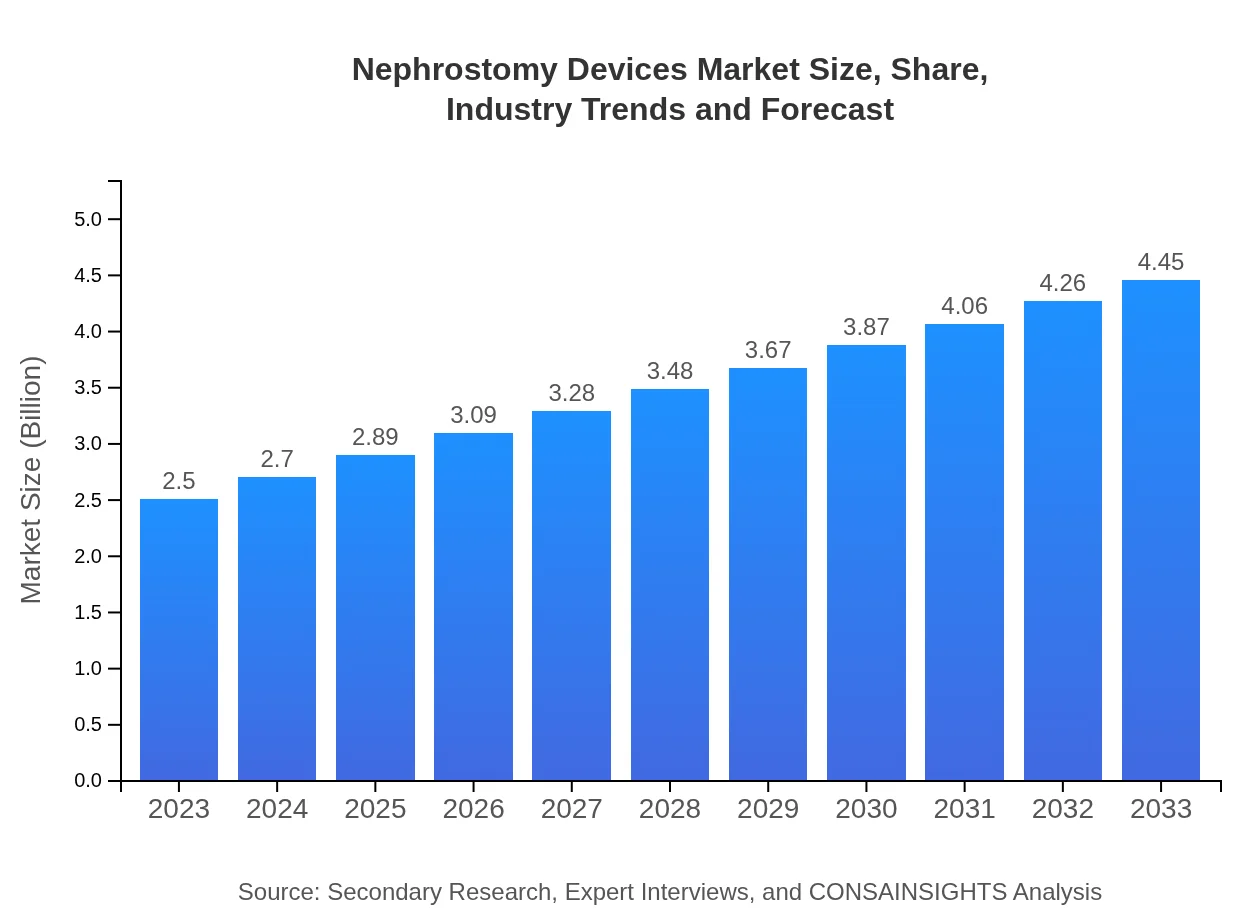
| 2023 Market Size | $2.50 Billion |
| CAGR (2023-2033) | 5.8% |
| 2033 Market Size | $4.45 Billion |
| Top Companies | Boston Scientific Corporation, Medtronic plc, Bard Medical, Cook Medical, Coloplast A/S |
| Last Modified Date | 31 January 2026 |
Nephrostomy Devices Market Overview
Customize Nephrostomy Devices Market Report market research report
- ✔ Get in-depth analysis of Nephrostomy Devices market size, growth, and forecasts.
- ✔ Understand Nephrostomy Devices's regional dynamics and industry-specific trends.
- ✔ Identify potential applications, end-user demand, and growth segments in Nephrostomy Devices
What is the Market Size & CAGR of Nephrostomy Devices market in 2023?
Nephrostomy Devices Industry Analysis
Nephrostomy Devices Market Segmentation and Scope
Tell us your focus area and get a customized research report.
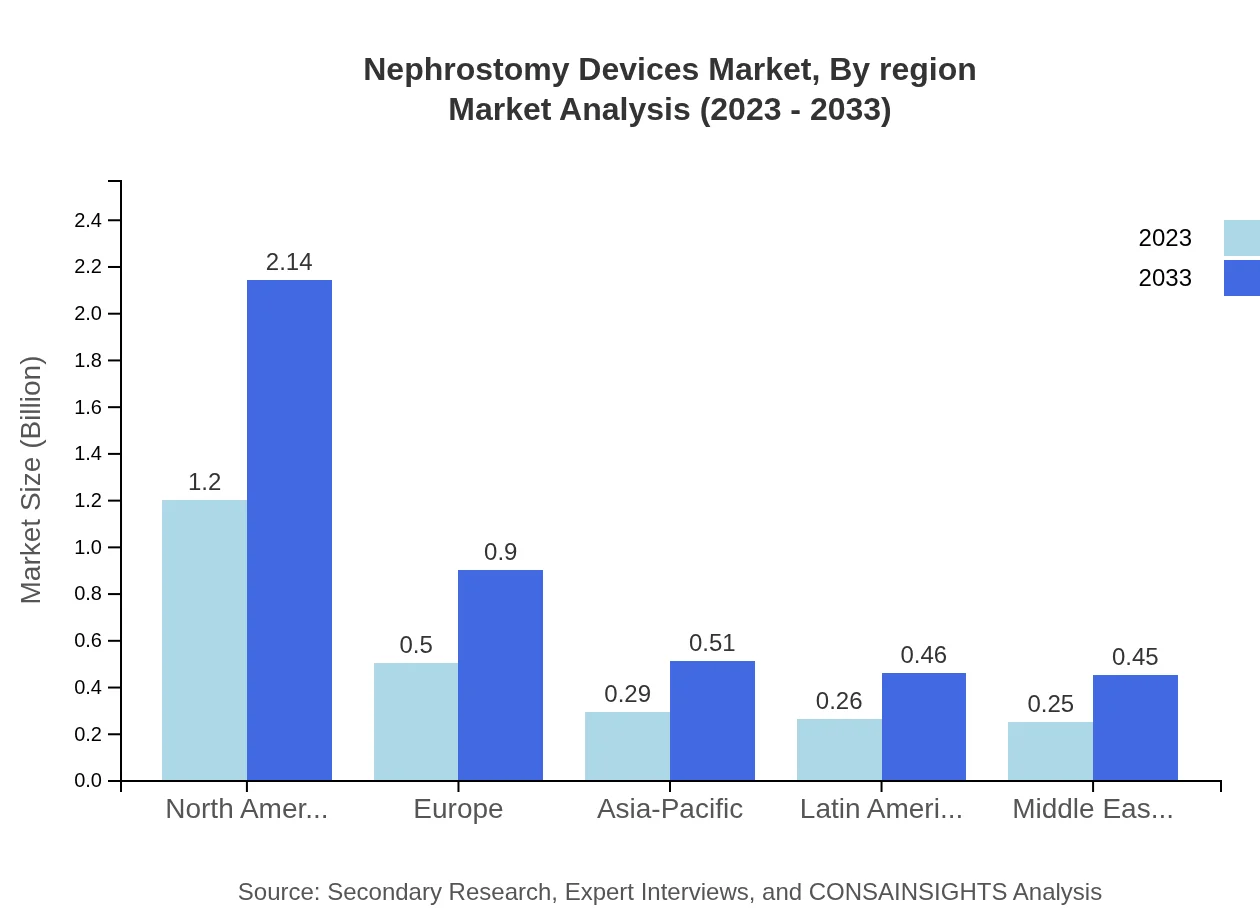
Nephrostomy Devices Market Analysis Report by Region
Europe Nephrostomy Devices Market Report:
In Europe, the market is anticipated to expand from $0.89 billion in 2023 to $1.59 billion by 2033. The ongoing advancements in treatment protocols and increased awareness regarding kidney health are key drivers. Countries like Germany and the UK are frontrunners in this growth.Asia Pacific Nephrostomy Devices Market Report:
In Asia Pacific, the nephrostomy devices market is anticipated to grow from $0.41 billion in 2023 to $0.73 billion by 2033. Factors contributing to this growth include increased healthcare expenditure, an aging population, and a rise in chronic kidney disease prevalence. Countries like Japan and Australia are leading the market due to advanced healthcare facilities.North America Nephrostomy Devices Market Report:
North America is expected to maintain its dominance, with market size projected to grow from $0.85 billion in 2023 to $1.51 billion by 2033. High healthcare spending, along with innovation in nephrostomy technologies, significantly drives this market. The U.S. is the largest contributor due to advanced medical practices and a robust healthcare sector.South America Nephrostomy Devices Market Report:
The South American market is relatively smaller, projected to increase from $0.03 billion in 2023 to $0.05 billion by 2033. The growth will predominantly be driven by improvements in healthcare access and rising awareness about nephrology.Middle East & Africa Nephrostomy Devices Market Report:
The Middle East and Africa market size is projected to grow from $0.32 billion to $0.56 billion by 2033. The gradual improvements in medical facilities and growing healthcare investments in emerging economies are pivotal for market growth in this region.Tell us your focus area and get a customized research report.
Nephrostomy Devices Market Analysis By Device Type
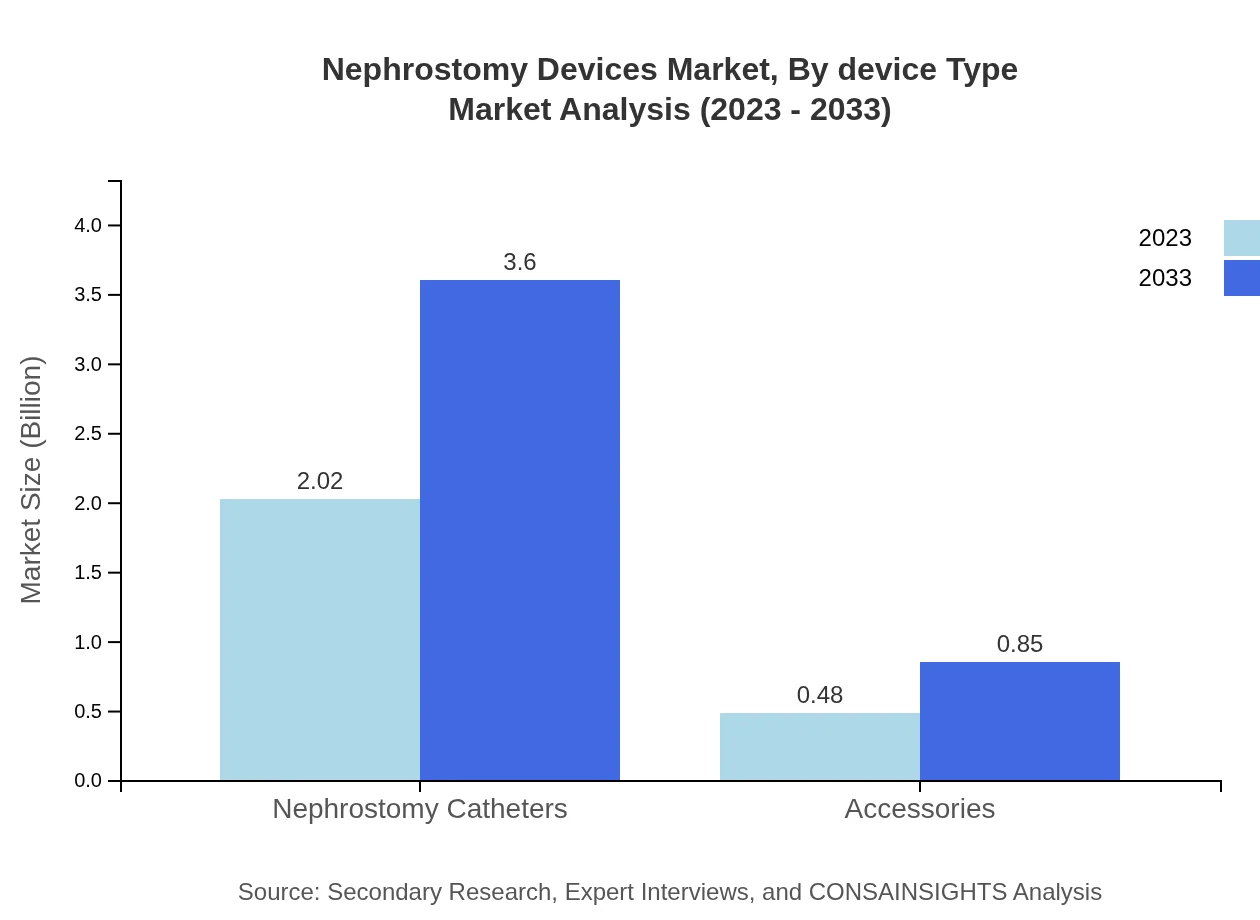
The nephrostomy devices market showcases key product types, with nephrostomy catheters accounting for a substantial share. In 2023, nephrostomy catheters comprised approximately $2.02 billion of the total market, projected to grow to $3.60 billion by 2033, reflecting around 80.91% market share by this product type.
Nephrostomy Devices Market Analysis By Application
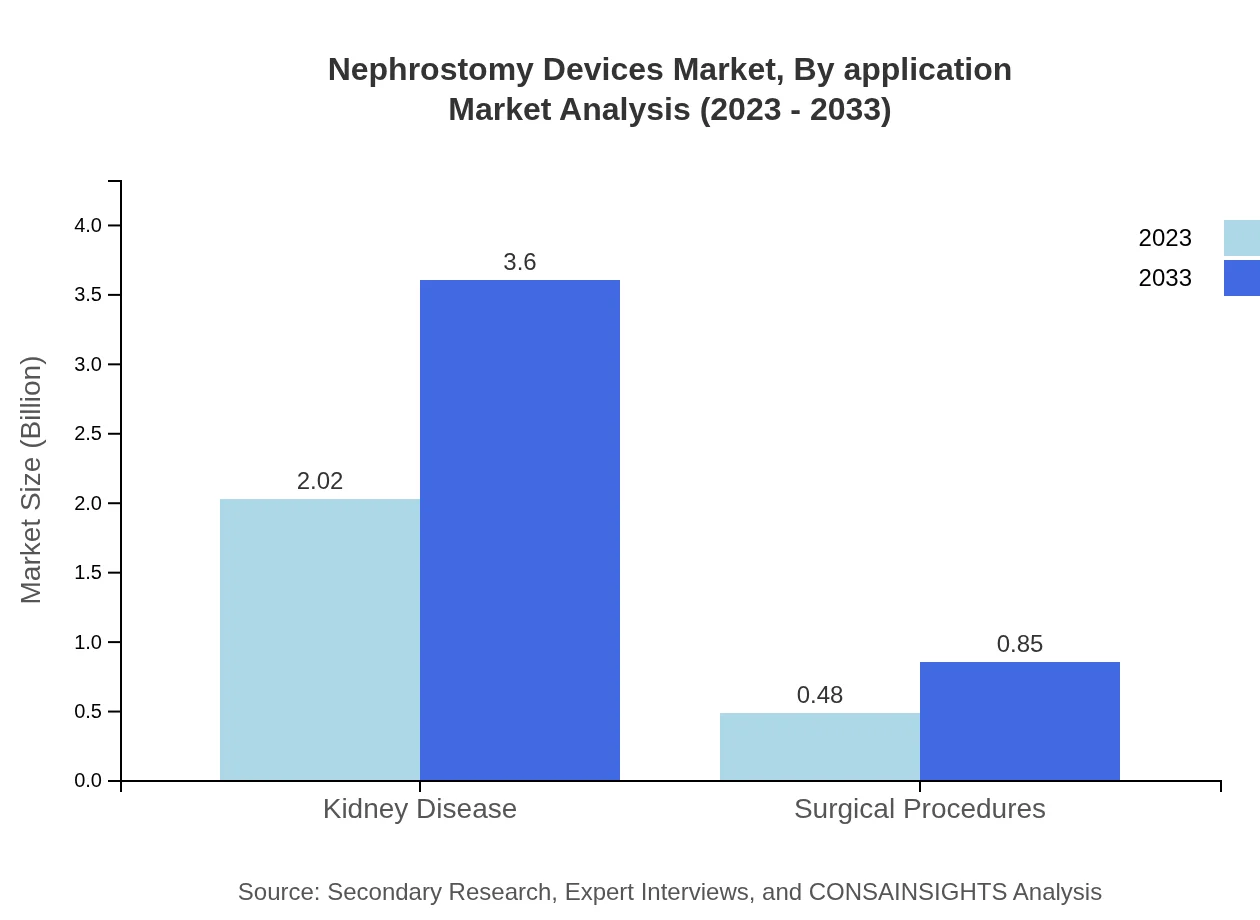
Focusing on application, the market is dominated by the kidney disease segment, estimated at $2.02 billion in 2023 and expected to reach $3.60 billion by 2033. This segment is critical, representing an 80.91% market share due to the rising prevalence of kidney disorders requiring such drainage procedures.
Nephrostomy Devices Market Analysis By End User
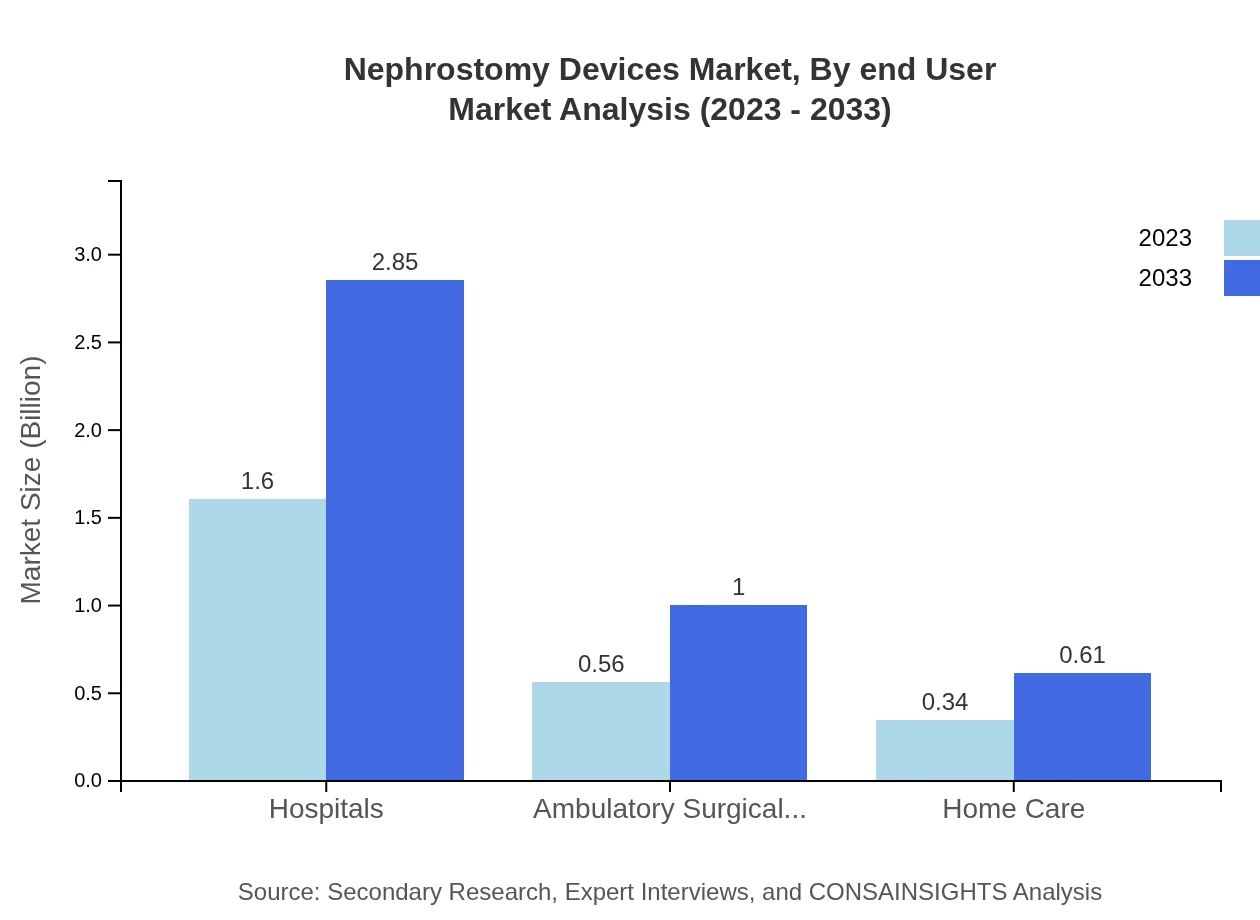
In terms of end-user segmentation, hospitals lead the market with a size of $1.60 billion in 2023, expected to expand to $2.85 billion by 2033. The hospital segment accounts for 64.01% of the total market, driven by the high volume of surgical procedures and increasing hospital admissions for renal treatments.
Nephrostomy Devices Market Analysis By Region
The regional analysis highlights North America maintaining its position as the market leader, followed by Europe and Asia Pacific, which are both witnessing rapid growth due to technological advancements and increasing healthcare access. Each region presents unique opportunities and challenges that can influence market trajectories significantly.
Nephrostomy Devices Market Trends and Future Forecast
Tell us your focus area and get a customized research report.
Global Market Leaders and Top Companies in Nephrostomy Devices Industry
Boston Scientific Corporation:
A renowned leader in the medical device industry providing comprehensive solutions, including nephrostomy devices and related technologies.Medtronic plc:
A global healthcare leader offering a broad range of products, including innovative nephrostomy devices, contributing to improved patient outcomes.Bard Medical:
Specializing in urology, Bard Medical provides a variety of nephrostomy and drainage devices critical for renal health management.Cook Medical:
Cook Medical is dedicated to advancing medical technology in various sectors, including nephrostomy devices, offering state-of-the-art patient care.Coloplast A/S:
A leading international company providing personal healthcare products and solutions, including nephrostomy devices, to enhance quality of life.We're grateful to work with incredible clients.









FAQs
What is the market size of nephrostomy devices?
The nephrostomy devices market is valued at approximately $2.5 billion in 2023, with a projected growth rate (CAGR) of 5.8% through 2033, indicating robust expansion and increasing demand.
What are the key market players or companies in this nephrostomy devices industry?
Key players in the nephrostomy devices market include major medical device manufacturers focused on renal care, such as Medtronic, Boston Scientific, and Cook Medical, along with emerging companies that specialize in innovative nephrology solutions.
What are the primary factors driving the growth in the nephrostomy devices industry?
The growth in the nephrostomy devices industry is driven by rising incidences of kidney-related disorders, technological advancements in device design, increased awareness of renal health, and an expanding patient population requiring such interventions.
Which region is the fastest Growing in the nephrostomy devices market?
The fastest-growing region in the nephrostomy devices market is North America, projected to grow from $0.85 billion in 2023 to $1.51 billion in 2033, followed closely by Europe from $0.89 billion to $1.59 billion.
Does ConsaInsights provide customized market report data for the nephrostomy devices industry?
Yes, ConsaInsights offers customized market report data tailored to specific client needs in the nephrostomy devices industry, ensuring comprehensive insights tailored to various stakeholders in the healthcare industry.
What deliverables can I expect from this nephrostomy devices market research project?
Expected deliverables from the nephrostomy devices market research project include detailed market analysis, competitive landscape insights, segmentation data, regional trends, and forecasts, along with actionable recommendations for stakeholders.
What are the market trends of nephrostomy devices?
Current market trends in nephrostomy devices include a shift towards minimally invasive procedures, an increase in home-care solutions, and the rising integration of digital health technologies to enhance patient monitoring and care.