Netupitantpalonosetron Fdc Market Report
Published Date: 31 January 2026 | Report Code: netupitantpalonosetron-fdc
Netupitantpalonosetron Fdc Market Size, Share, Industry Trends and Forecast to 2033
This report provides comprehensive insights into the Netupitantpalonosetron Fdc market, analyzing market size, growth trends, key players, and forecasted developments from 2023 to 2033. It encompasses industry analysis, segmentation, regional insights, and future market trends.
| Metric | Value |
|---|---|
| Study Period | 2023 - 2033 |
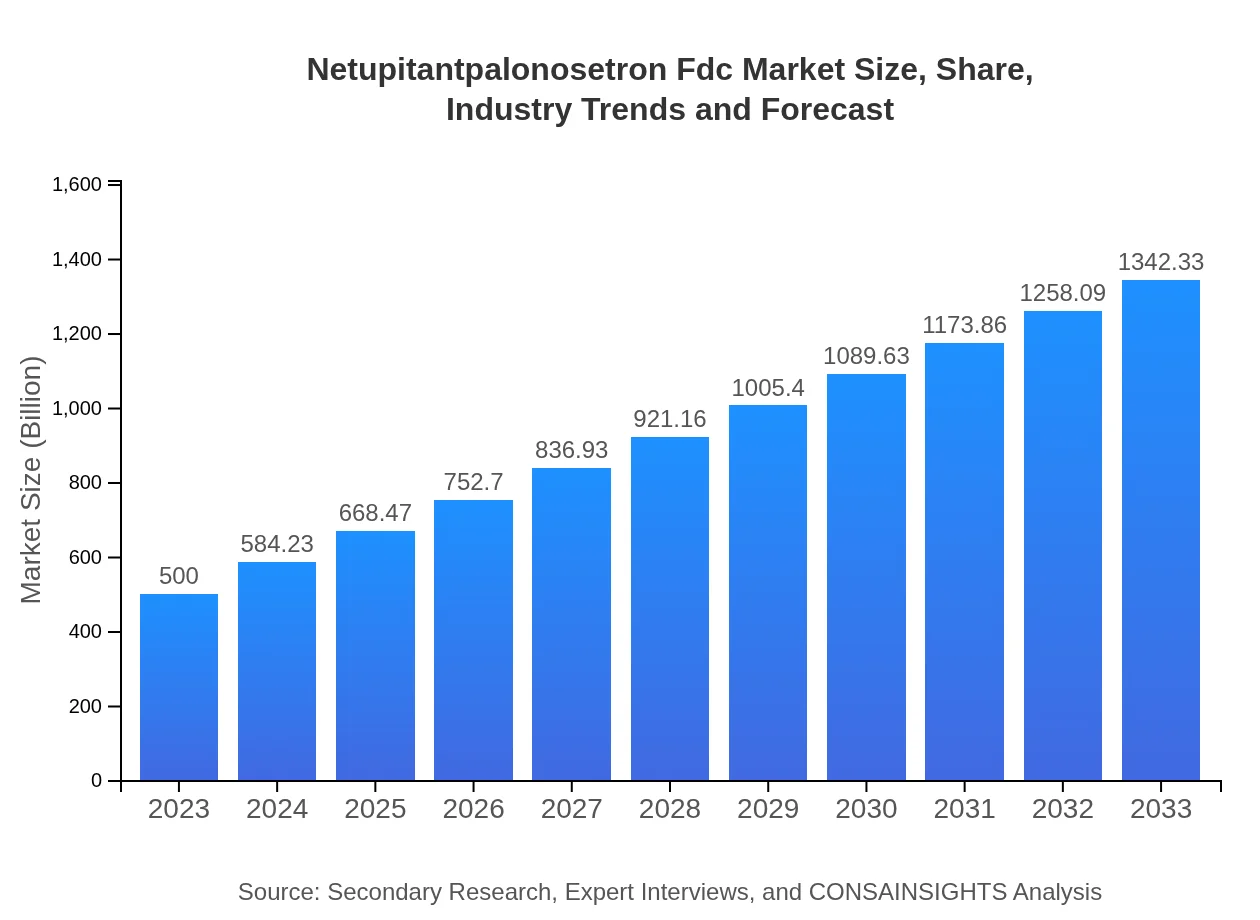
| 2023 Market Size | $500.00 Million |
| CAGR (2023-2033) | 10% |
| 2033 Market Size | $1342.33 Million |
| Top Companies | Merck & Co., Helsinn Healthcare SA, Teva Pharmaceutical Industries Ltd. |
| Last Modified Date | 31 January 2026 |
Netupitantpalonosetron Fdc Market Overview
Customize Netupitantpalonosetron Fdc Market Report market research report
- ✔ Get in-depth analysis of Netupitantpalonosetron Fdc market size, growth, and forecasts.
- ✔ Understand Netupitantpalonosetron Fdc's regional dynamics and industry-specific trends.
- ✔ Identify potential applications, end-user demand, and growth segments in Netupitantpalonosetron Fdc
What is the Market Size & CAGR of Netupitantpalonosetron Fdc market in 2023?
Netupitantpalonosetron Fdc Industry Analysis
Netupitantpalonosetron Fdc Market Segmentation and Scope
Tell us your focus area and get a customized research report.
Netupitantpalonosetron Fdc Market Analysis Report by Region
Europe Netupitantpalonosetron Fdc Market Report:
The European market for Netupitantpalonosetron Fdc is expected to expand from $130.10 million in 2023 to $349.27 million by 2033. This growth is driven by stringent regulatory approvals and a strong emphasis on treating nausea-related disorders in oncology.Asia Pacific Netupitantpalonosetron Fdc Market Report:
The Asia Pacific region is projected to experience robust growth, with market size expected to rise from $102.35 million in 2023 to $274.77 million by 2033. This growth is fueled by increasing patient populations, expanding healthcare infrastructures, and heightened awareness regarding nausea management across emerging economies.North America Netupitantpalonosetron Fdc Market Report:
North America continues to dominate the Netupitantpalonosetron Fdc market, with a size forecast from $181.85 million in 2023 to $488.20 million by 2033. The region benefits from advanced healthcare systems, high treatment adherence rates, and a focus on innovative therapeutics.South America Netupitantpalonosetron Fdc Market Report:
In South America, the market size for Netupitantpalonosetron Fdc is anticipated to grow from $23.10 million in 2023 to $62.02 million by 2033. Growth is supported by government initiatives to improve healthcare access and investments in pharmaceuticals, particularly in Brazil and Argentina.Middle East & Africa Netupitantpalonosetron Fdc Market Report:
The Middle East and Africa region's market size for Netupitantpalonosetron Fdc is set to grow from $62.60 million in 2023 to $168.06 million by 2033, supported by rising healthcare investments and a growing demand for effective nausea management solutions.Tell us your focus area and get a customized research report.
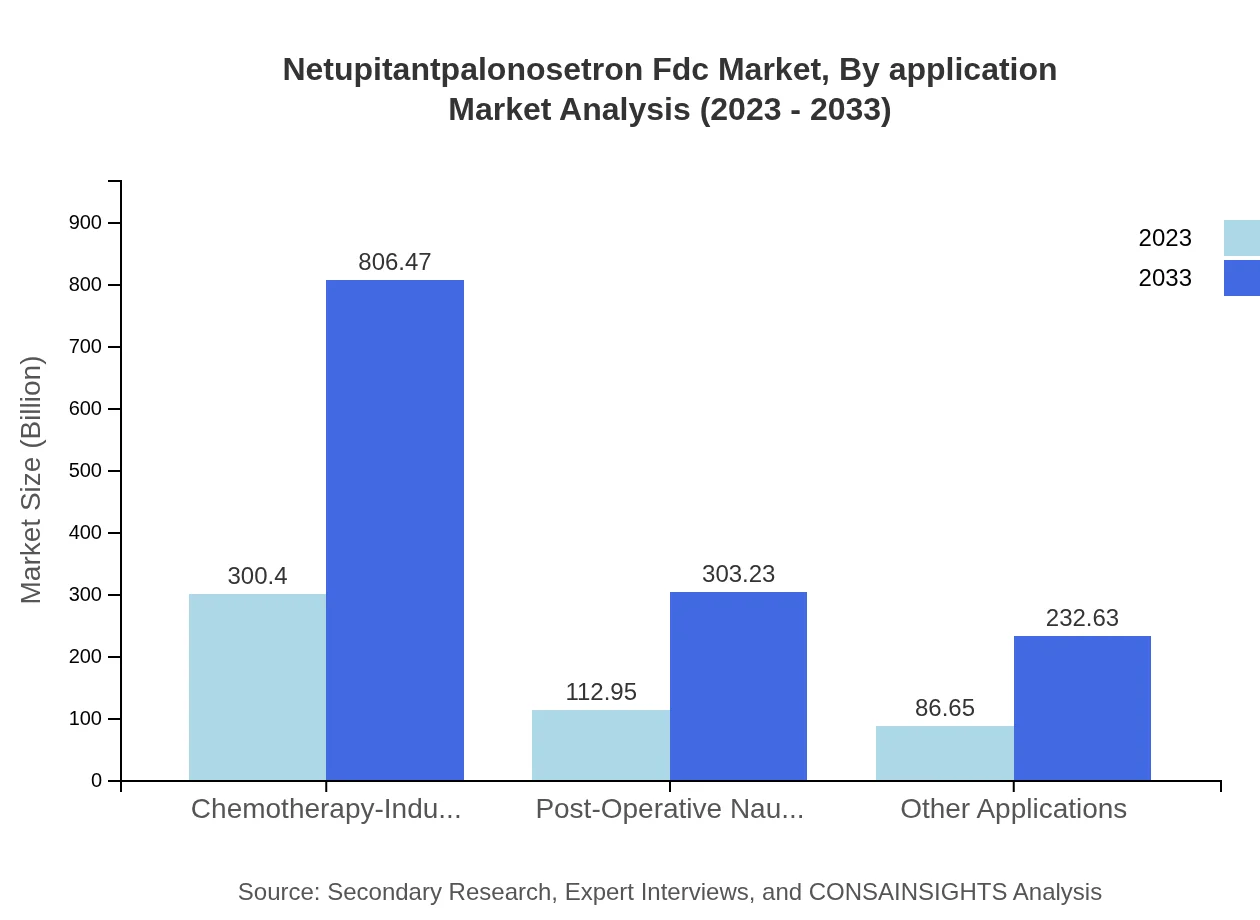
Netupitantpalonosetron Fdc Market Analysis By Application
The Netupitant/Palonosetron FDC market, segmented by application, reveals a significant focus on chemotherapy-induced nausea and vomiting, which accounts for approximately 60.08% of the total market share in both 2023 and 2033. Post-operative nausea and vomiting applications represent another crucial segment with significant growth potential, projected to reach 22.59% share as postoperative procedures rise.
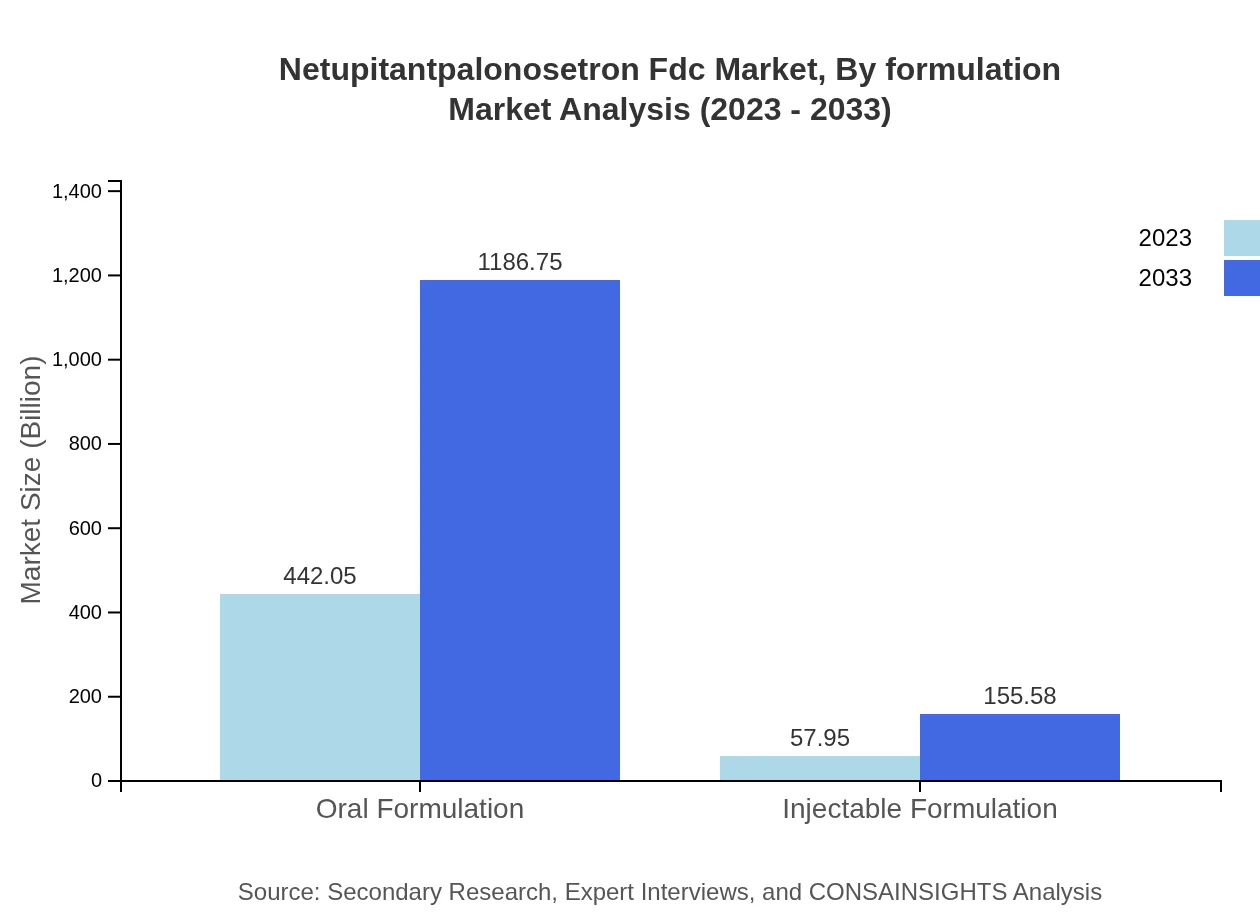
Netupitantpalonosetron Fdc Market Analysis By Formulation
In terms of formulation, the oral formulation segment is leading with a massive market portion of 88.41%, expected to grow from $442.05 million in 2023 to $1,186.75 million by 2033. Injectable formulations, holding an 11.59% share, are projected to see moderate growth, reflecting patient preferences for oral delivery methods unless specific conditions necessitate injections.
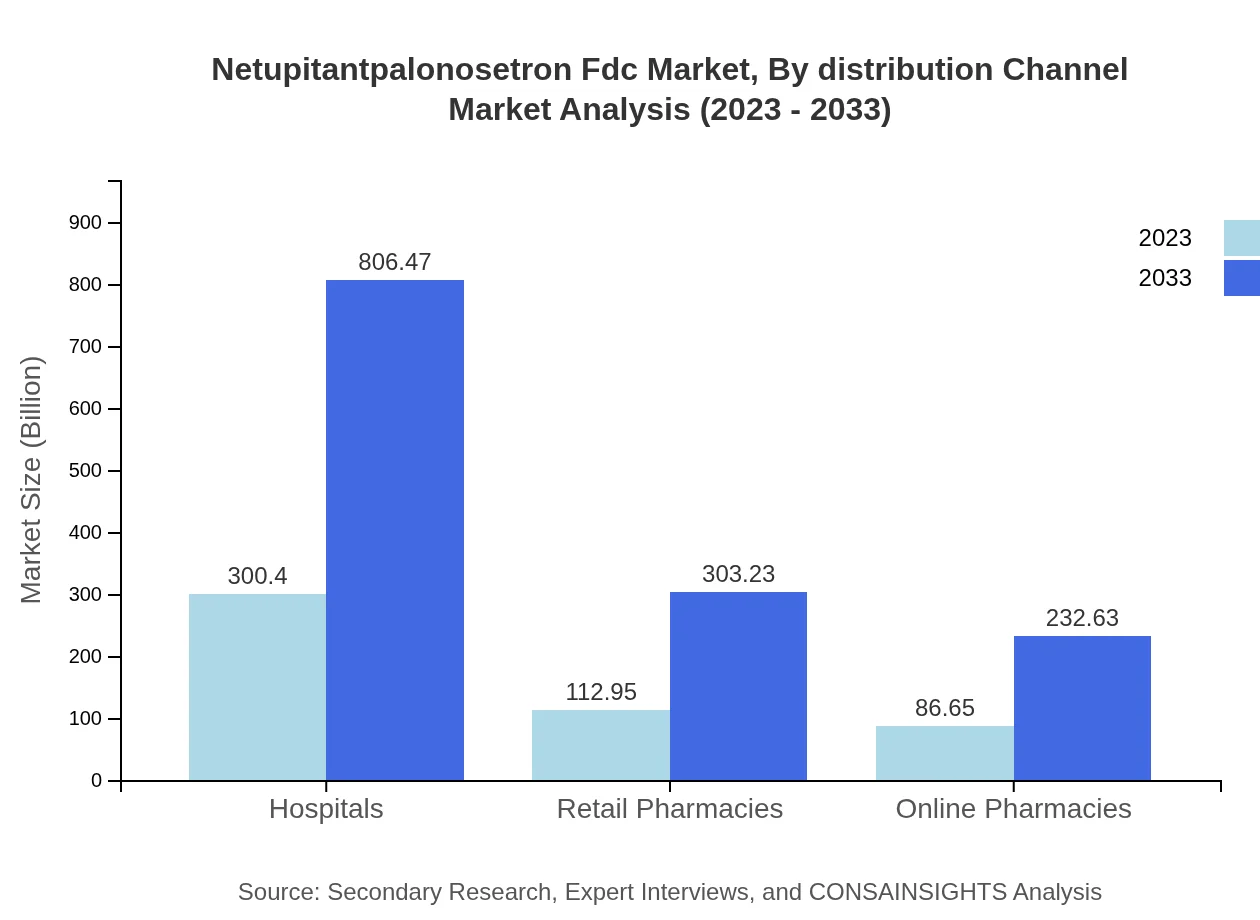
Netupitantpalonosetron Fdc Market Analysis By Distribution Channel
Distribution of Netupitantpalonosetron Fdc is primarily dominated by hospitals, which account for 60.08% of total market share in both years analyzed. Retail pharmacies and online pharmacies follow at around 22.59% and 17.33% share respectively. The rise of telemedicine is expected to elevate the volume of online purchases, reflecting a trend towards convenience.
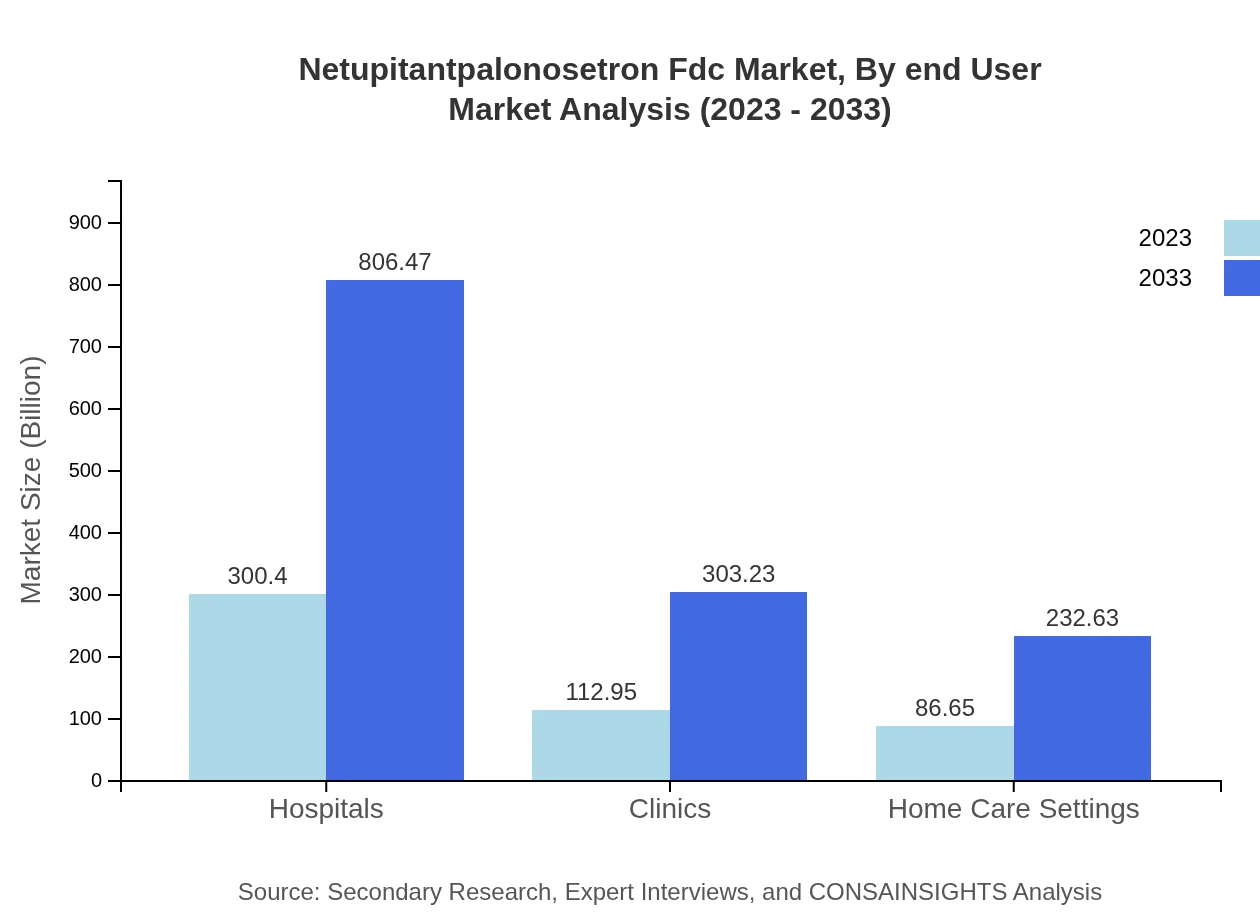
Netupitantpalonosetron Fdc Market Analysis By End User
The end-user analysis highlights hospitals as the predominant setting for netupitantpalonosetron Fdc usage, with a gradual uptick expected in home care settings as patient preferences shift. Advances in telehealth and remote monitoring are further contributing to the significance of home care applications.
Netupitantpalonosetron Fdc Market Trends and Future Forecast
Tell us your focus area and get a customized research report.
Global Market Leaders and Top Companies in Netupitantpalonosetron Fdc Industry
Merck & Co.:
Merck is a leading pharmaceutical company specializing in improving health through innovation. Their investment in research and development has placed them at the forefront of the Netupitantpalonosetron Fdc market.Helsinn Healthcare SA:
Helsinn is recognized for its comprehensive portfolio of supportive care products for patients undergoing cancer treatment, advocating for better patient outcomes.Teva Pharmaceutical Industries Ltd.:
Teva is a global leader in generic and specialty medications, thereby significantly impacting the availability and affordability of Netupitantpalonosetron Fdc therapies.We're grateful to work with incredible clients.









FAQs
What is the market size of netupitantpalonosetron Fdc?
The market size of netupitantpalonosetron-fdc is projected to reach 500 million by 2033, with a compound annual growth rate (CAGR) of 10% over the forecast period. This indicates robust growth potential within this pharmaceutical segment.
What are the key market players or companies in this netupitantpalonosetron Fdc industry?
Key players in the netupitantpalonosetron-fdc market include major pharmaceutical companies specializing in oncology and supportive care. These companies focus on innovation and product development to capture a significant market share.
What are the primary factors driving the growth in the netupitantpalonosetron Fdc industry?
Growth drivers in the netupitantpalonosetron-fdc industry include increasing prevalence of cancer, rising demand for effective antiemetics, advancements in drug formulations, and supportive healthcare policies that enhance patient access to innovative therapies.
Which region is the fastest Growing in the netupitantpalonosetron Fdc?
The Asia-Pacific region is the fastest-growing market for netupitantpalonosetron-fdc, with a projected market increase from 102.35 million in 2023 to 274.77 million by 2033, reflecting a strong demand for effective cancer treatments and supportive therapies.
Does ConsaInsights provide customized market report data for the netupitantpalonosetron Fdc industry?
Yes, ConsaInsights offers customized market report data for the netupitantpalonosetron-fdc industry, providing tailored insights and analyses that address specific client needs and strategic objectives.
What deliverables can I expect from this netupitantpalonosetron Fdc market research project?
Deliverables from the netupitantpalonosetron-fdc market research project include comprehensive reports, market analysis data, growth projections, competitive landscape assessments, and tailored insights for strategic planning and decision-making.
What are the market trends of netupitantpalonosetron Fdc?
Current market trends for netupitantpalonosetron-fdc include an increasing focus on oral formulations, expanding applications in various settings, and heightened investment in research and development to improve treatment efficacy for chemotherapy-induced nausea.