Neurovascular Devices Interventional Neurology Market Report
Published Date: 31 January 2026 | Report Code: neurovascular-devices-interventional-neurology
Neurovascular Devices Interventional Neurology Market Size, Share, Industry Trends and Forecast to 2033
This report provides an in-depth analysis of the Neurovascular Devices Interventional Neurology market, covering market size, growth trends, segmentation, and forecasts from 2023 to 2033. Insights on key players, technological advancements, and regional dynamics are included to inform strategic decision-making.
| Metric | Value |
|---|---|
| Study Period | 2023 - 2033 |
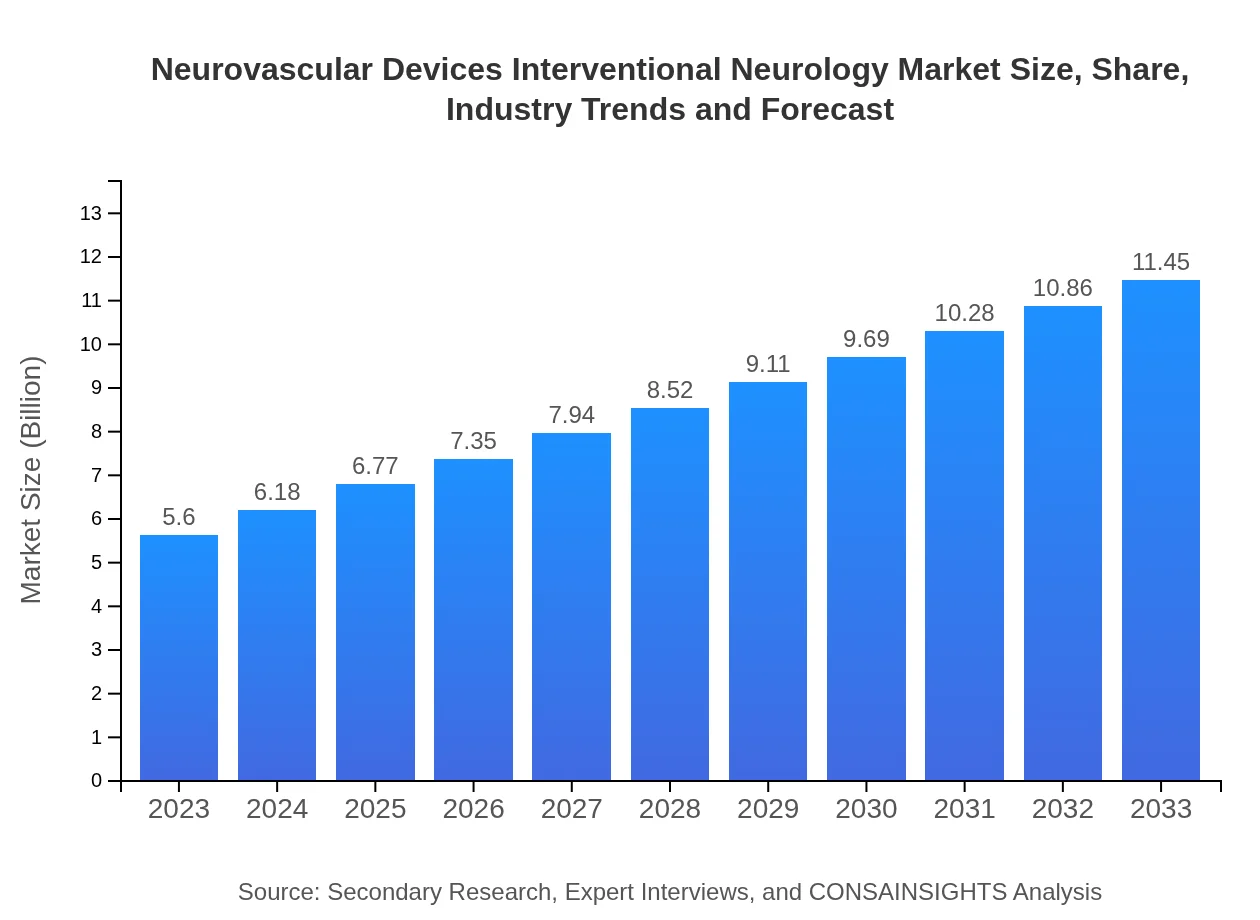
| 2023 Market Size | $5.60 Billion |
| CAGR (2023-2033) | 7.2% |
| 2033 Market Size | $11.45 Billion |
| Top Companies | Medtronic , Stryker , Penumbra, Inc., Johnson & Johnson, Boston Scientific |
| Last Modified Date | 31 January 2026 |
Neurovascular Devices Interventional Neurology Market Overview
Customize Neurovascular Devices Interventional Neurology Market Report market research report
- ✔ Get in-depth analysis of Neurovascular Devices Interventional Neurology market size, growth, and forecasts.
- ✔ Understand Neurovascular Devices Interventional Neurology's regional dynamics and industry-specific trends.
- ✔ Identify potential applications, end-user demand, and growth segments in Neurovascular Devices Interventional Neurology
What is the Market Size & CAGR of Neurovascular Devices Interventional Neurology market in 2023?
Neurovascular Devices Interventional Neurology Industry Analysis
Neurovascular Devices Interventional Neurology Market Segmentation and Scope
Tell us your focus area and get a customized research report.
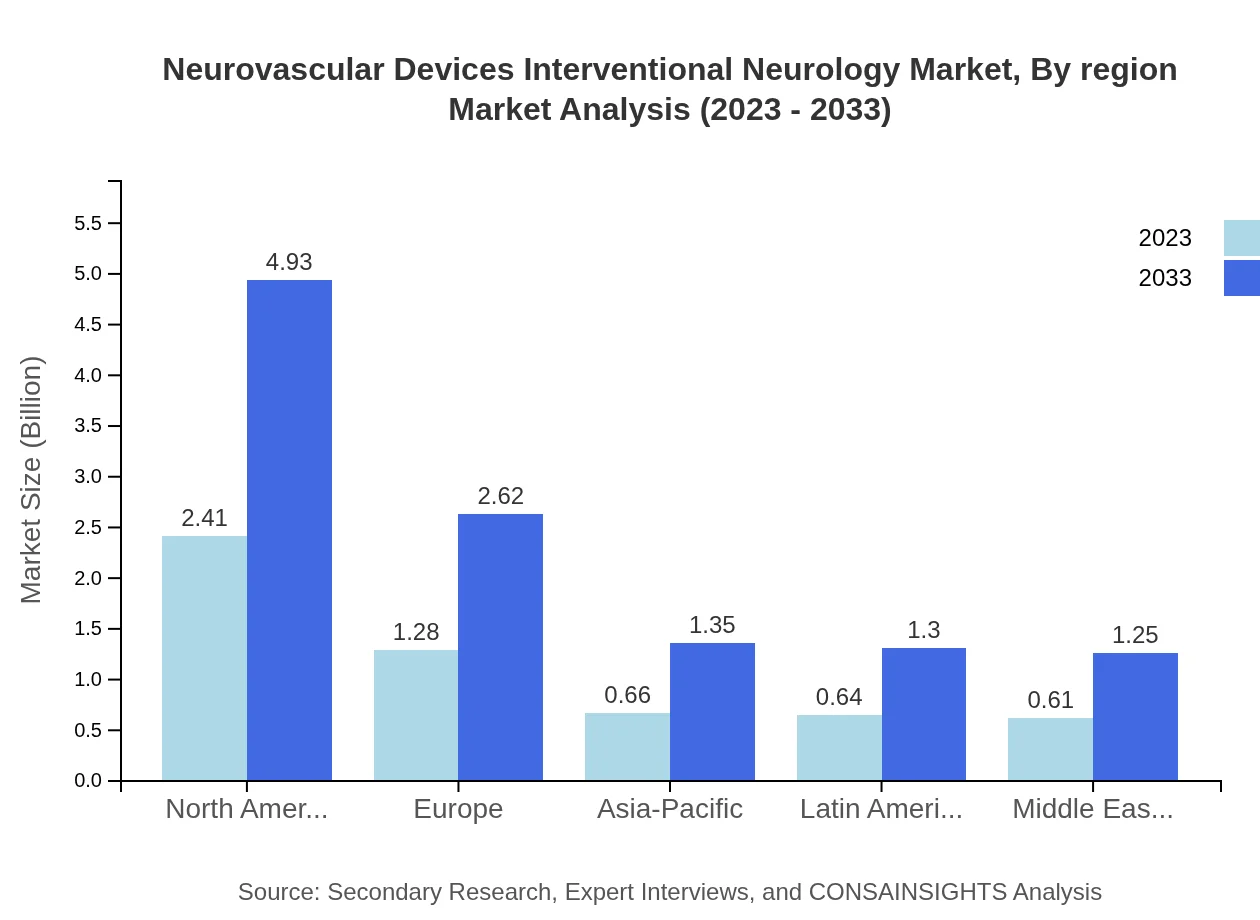
Neurovascular Devices Interventional Neurology Market Analysis Report by Region
Europe Neurovascular Devices Interventional Neurology Market Report:
In Europe, the market is set to grow from $1.92 billion in 2023 to $3.92 billion by 2033. The increasing demand for innovative treatment solutions and the presence of key industry players are propelling this growth, along with regulatory support for medical advancements.Asia Pacific Neurovascular Devices Interventional Neurology Market Report:
In 2023, the Neurovascular Devices market in the Asia Pacific is valued at approximately $0.89 billion and is expected to grow to $1.83 billion by 2033. This growth can be attributed to rising healthcare expenditure, increasing awareness about stroke treatment options, and a growing elderly population.North America Neurovascular Devices Interventional Neurology Market Report:
The North American market stands as a major contributor, with an estimated size of $1.96 billion in 2023, expanding to $4.00 billion by 2033. Factors driving this growth include high prevalence of neurovascular diseases, rapid adoption of advanced therapies, and significant healthcare spending.South America Neurovascular Devices Interventional Neurology Market Report:
South America’s Neurovascular Devices market is anticipated to grow from $0.10 billion in 2023 to about $0.21 billion in 2033. Growth drivers include improving healthcare infrastructure and enhanced access to advanced medical technologies.Middle East & Africa Neurovascular Devices Interventional Neurology Market Report:
The Middle East and Africa market is projected to reach $1.49 billion by 2033, growing from $0.73 billion in 2023. The increasing focus on healthcare improvement and investments in state-of-the-art medical devices in the region are driving market advancements.Tell us your focus area and get a customized research report.
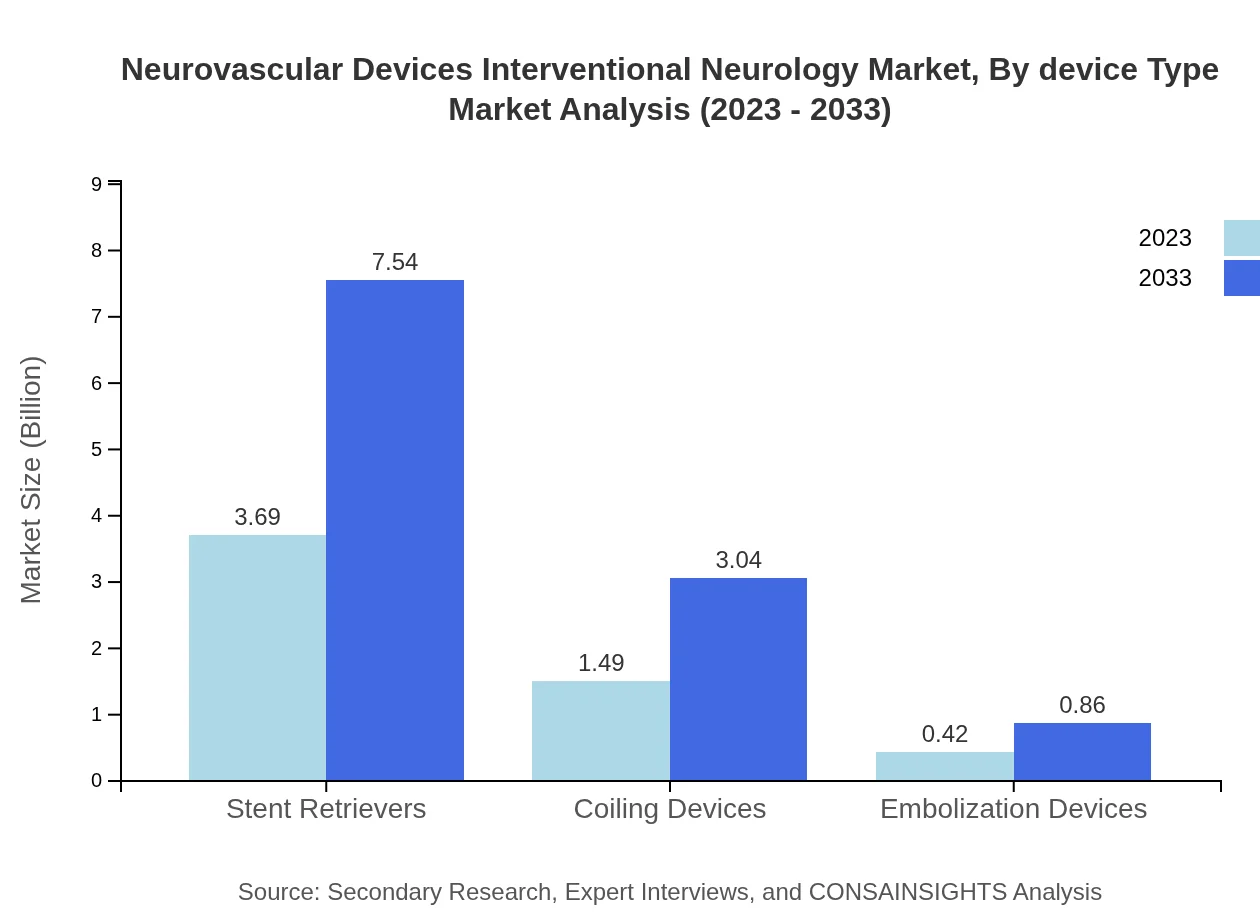
Neurovascular Devices Interventional Neurology Market Analysis By Device Type
The market is segmented by device type into stent retrievers, coiling devices, and embolization devices. Stent retrievers dominate the market due to their effectiveness in treating ischemic strokes, accounting for 65.88% market share in 2023, with expected growth indicating heightened reliance on these technologies for acute stroke management. Coiling devices and embolization devices also hold significant shares, addressing specific neurovascular conditions.
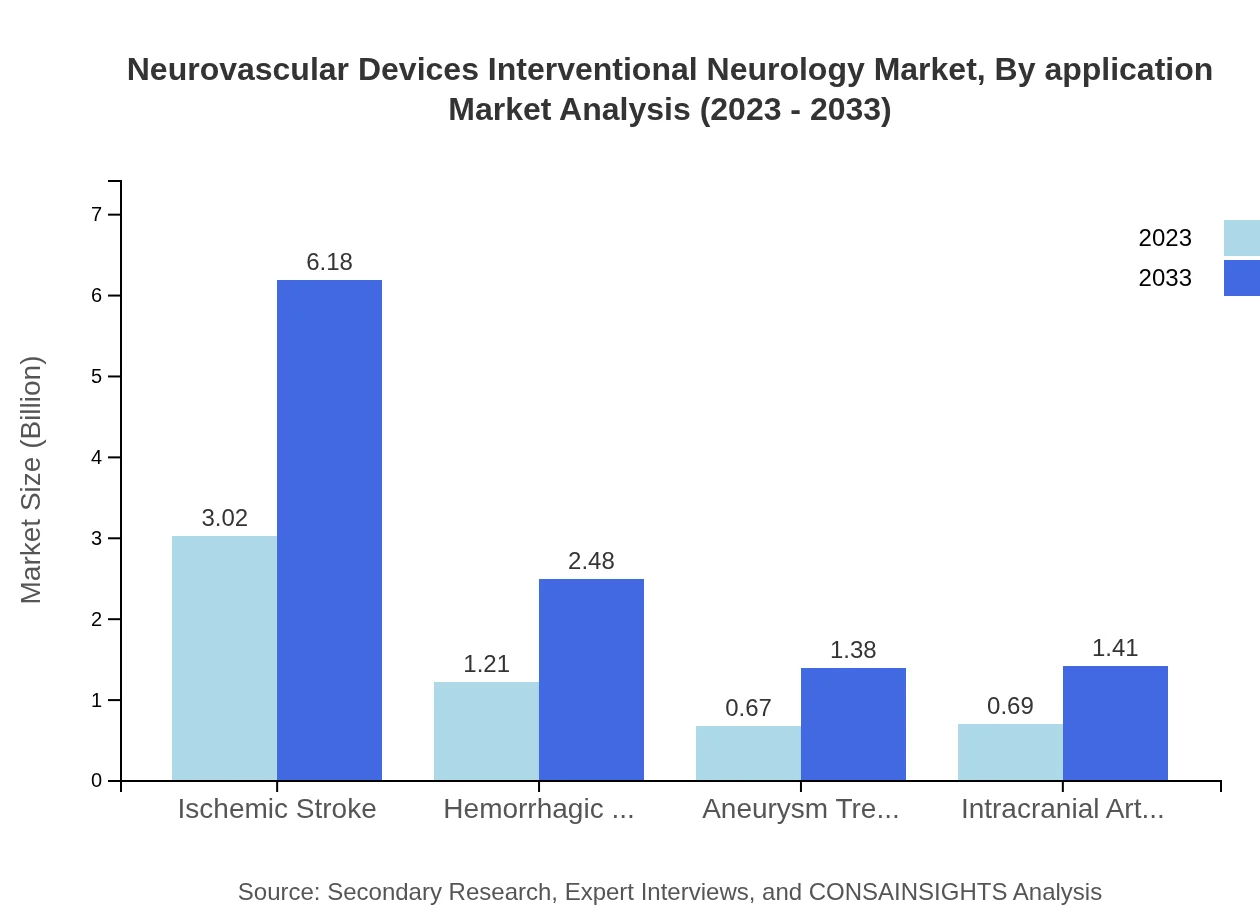
Neurovascular Devices Interventional Neurology Market Analysis By Application
Market segmentation by application highlights dominant areas such as ischemic stroke management, which holds a 54.01% share in 2023. Other applications include aneurysm treatment (12.03%) and intracranial arterial stenosis (12.28%). The rising incidence of these conditions coupled with the development of innovative treatment options fuels growth across these segments.
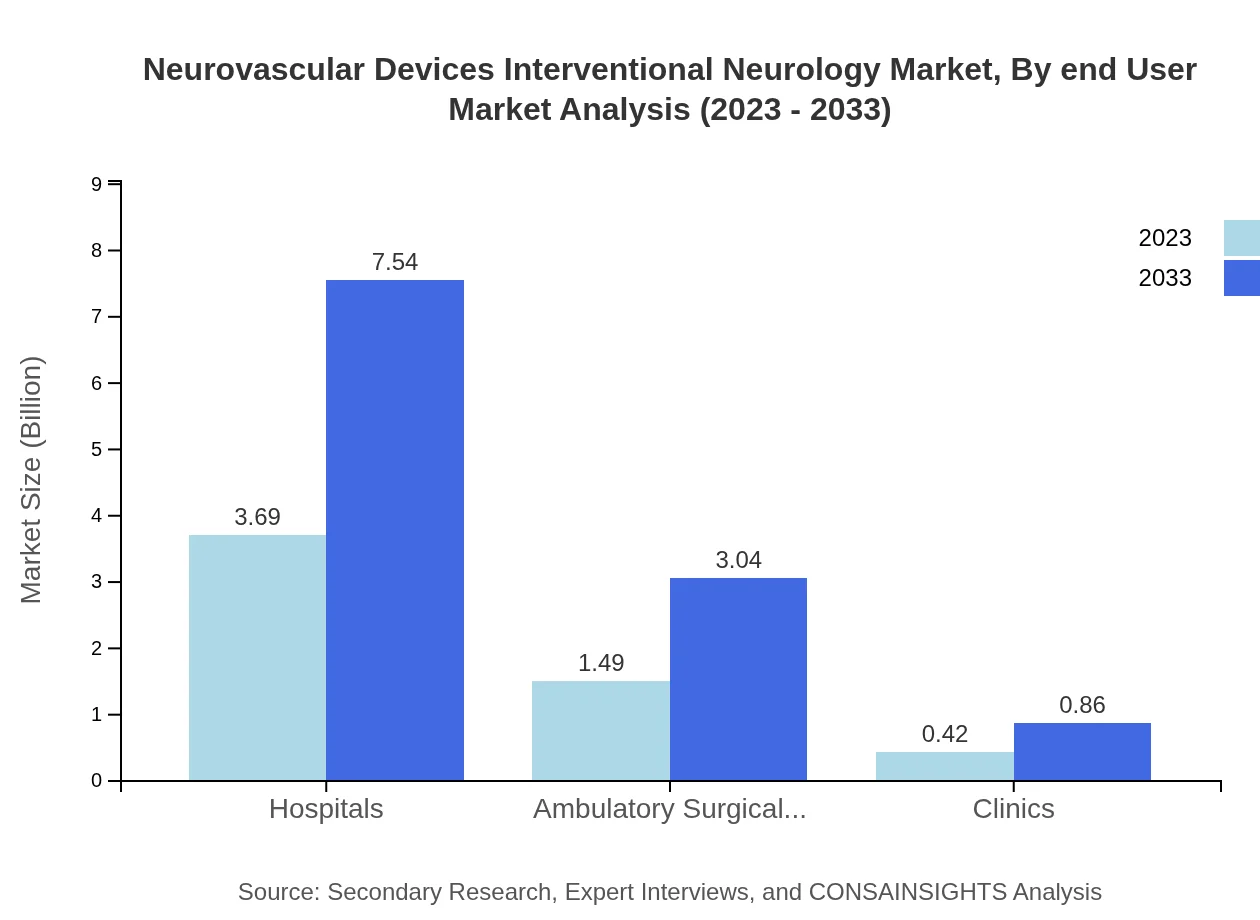
Neurovascular Devices Interventional Neurology Market Analysis By End User
Hospitals lead the end-user market with a 65.88% share in 2023, primarily due to their advanced capabilities in handling complex neurovascular procedures. Ambulatory surgical centers and clinics also contribute, with respective shares of 26.6% and 7.52%, underscoring a shifting trend towards outpatient services in the medical field.
Neurovascular Devices Interventional Neurology Market Analysis By Region
Regionally, North America occupies the largest share at 43.06% in 2023, followed by Europe (22.88%) and Asia-Pacific (11.8%). Each region is witnessing growth driven by increasing medical needs, technological advancements, and rising healthcare investment.
Neurovascular Devices Interventional Neurology Market Trends and Future Forecast
Tell us your focus area and get a customized research report.
Global Market Leaders and Top Companies in Neurovascular Devices Interventional Neurology Industry
Medtronic :
A leading global medical technology company specializing in innovative neurovascular solutions and devices for stroke management.Stryker :
Well-known for its advanced neurovascular products, Stryker focuses on minimally invasive approaches for the treatment of neurological conditions.Penumbra, Inc.:
A notable player in the market renowned for its focus on neurovascular interventions and the development of innovative tools for treating strokes.Johnson & Johnson:
Through its subsidiary, DePuy Synthes, it provides comprehensive neurovascular solutions focusing on market needs and innovation.Boston Scientific:
Famous for its advanced medical devices, Boston Scientific offers several innovative products specifically for the neurovascular field.We're grateful to work with incredible clients.









FAQs
What is the market size of neurovascular Devices Interventional Neurology?
The neurovascular devices interventional neurology market is projected to reach a size of $5.6 billion by 2033, growing at a CAGR of 7.2%. This growth reflects the increasing demand for advanced medical technologies in neurological interventions.
What are the key market players or companies in this neurovascular Devices Interventional Neurology industry?
Key players in the neurovascular devices interventional neurology market include international giants such as Medtronic, Abbott Laboratories, Johnson & Johnson, Stryker Corporation, and Siemens Healthineers. Their innovation and extensive distribution networks drive market competition.
What are the primary factors driving the growth in the neurovascular Devices Interventional Neurology industry?
Growth drivers include the rising prevalence of neurological disorders such as strokes, advancements in minimally invasive techniques, increasing demand for interventional procedures, and the growing awareness of and investments in neurovascular health.
Which region is the fastest Growing in the neurovascular Devices Interventional Neurology?
The Asia Pacific region is the fastest-growing area in the neurovascular devices market, projected to grow from $0.89 billion in 2023 to $1.83 billion by 2033, driven by improved healthcare infrastructure and increasing patient awareness.
Does ConsaInsights provide customized market report data for the neurovascular Devices Interventional Neurology industry?
Yes, ConsaInsights offers customized market report data tailored specifically to the neurovascular devices interventional neurology industry, allowing clients to access detailed insights according to their specific needs and market focus.
What deliverables can I expect from this neurovascular Devices Interventional Neurology market research project?
Clients can expect comprehensive deliverables including market analysis reports, regional insights, competitive landscape summaries, trend analysis, and forecasts up to 2033, tailored according to specific market segments.
What are the market trends of neurovascular Devices Interventional Neurology?
Current trends include increasing investment in R&D for novel devices, the rise of robot-assisted surgeries, and the growing preference for outpatient procedures, reflecting a broader shift toward integrated care solutions.