Newborn Screening Market Report
Published Date: 31 January 2026 | Report Code: newborn-screening
Newborn Screening Market Size, Share, Industry Trends and Forecast to 2033
This report provides a comprehensive analysis of the Newborn Screening market, covering key insights and data from 2023 to 2033, including market size, growth projections, segmentation, and regional dynamics.
| Metric | Value |
|---|---|
| Study Period | 2023 - 2033 |
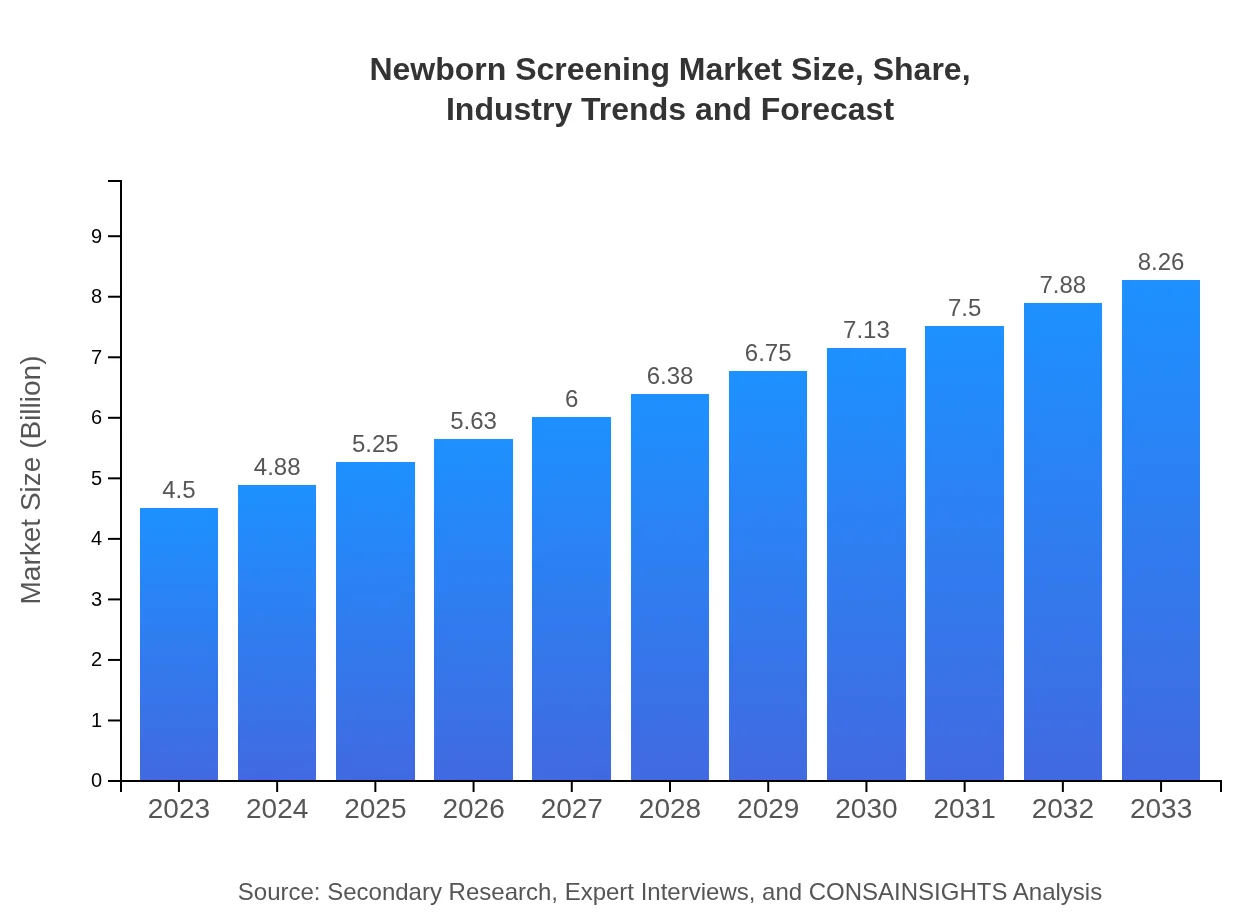
| 2023 Market Size | $4.50 Billion |
| CAGR (2023-2033) | 6.1% |
| 2033 Market Size | $8.26 Billion |
| Top Companies | PerkinElmer, Inc., Abbott Laboratories, Thermo Fisher Scientific, Agilent Technologies, Roche Diagnostics |
| Last Modified Date | 31 January 2026 |
Newborn Screening Market Overview
Customize Newborn Screening Market Report market research report
- ✔ Get in-depth analysis of Newborn Screening market size, growth, and forecasts.
- ✔ Understand Newborn Screening's regional dynamics and industry-specific trends.
- ✔ Identify potential applications, end-user demand, and growth segments in Newborn Screening
What is the Market Size & CAGR of the Newborn Screening market in 2023?
Newborn Screening Industry Analysis
Newborn Screening Market Segmentation and Scope
Tell us your focus area and get a customized research report.
Newborn Screening Market Analysis Report by Region
Europe Newborn Screening Market Report:
Europe is anticipated to grow significantly from $1.16 billion in 2023 to $2.12 billion by 2033 owing to stringent regulations promoting newborn health initiatives and increased healthcare expenditures.Asia Pacific Newborn Screening Market Report:
In the Asia Pacific region, the market is poised to grow significantly from $0.98 billion in 2023 to $1.79 billion in 2033. Factors driving growth include rising maternal and child health awareness and initiatives for early disease detection.North America Newborn Screening Market Report:
North America holds a substantial market share, projected to grow from $1.53 billion in 2023 to $2.81 billion in 2033, driven by advanced healthcare systems and high prevalence of congenital disorders.South America Newborn Screening Market Report:
South America is expected to see an increase from $0.23 billion in 2023 to $0.43 billion in 2033. The market is growing due to improved healthcare infrastructure and government-led screening programs.Middle East & Africa Newborn Screening Market Report:
The Middle East and Africa market is expected to grow from $0.60 billion in 2023 to $1.10 billion in 2033. This growth is attributed to increasing investments in health infrastructure and growing awareness regarding newborn screening.Tell us your focus area and get a customized research report.
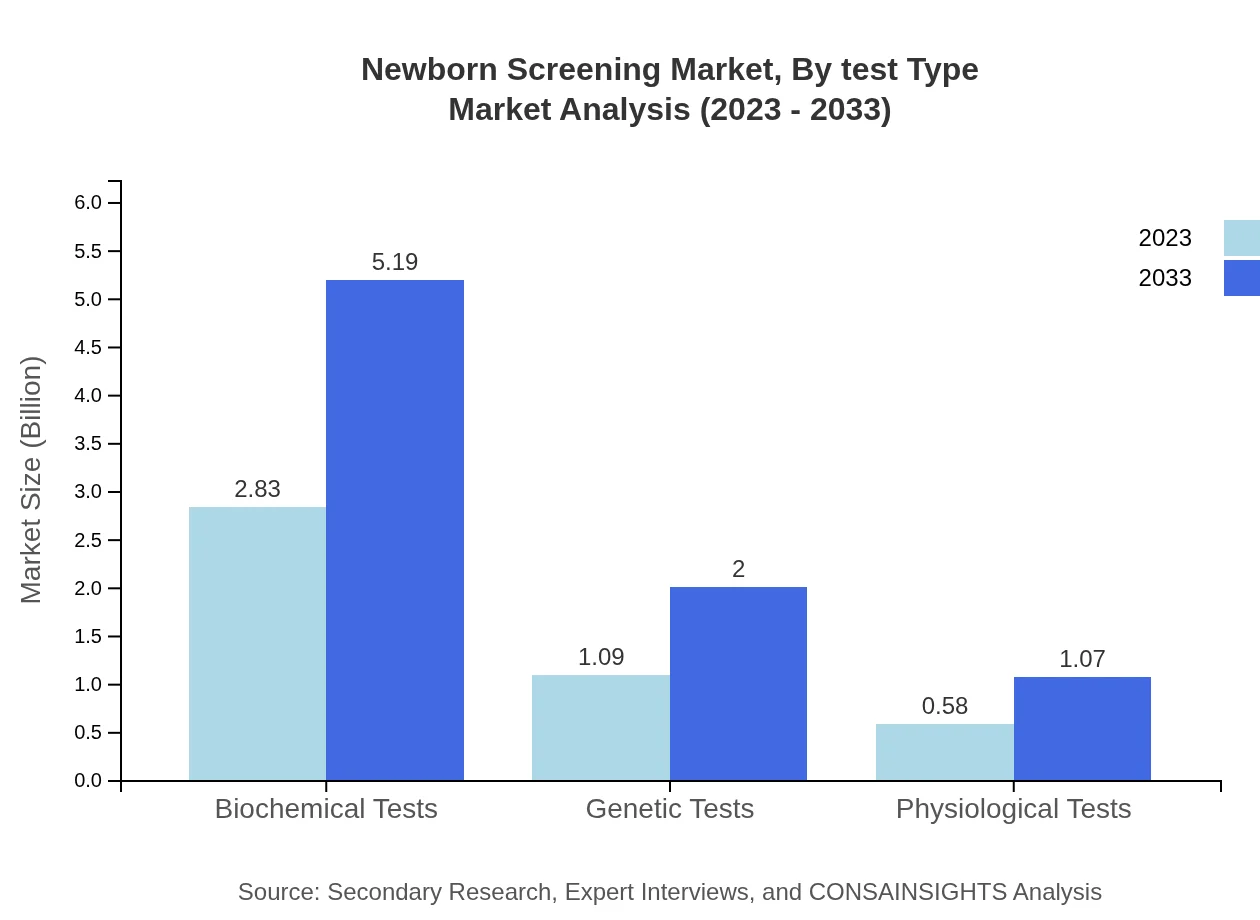
Newborn Screening Market Analysis By Test Type
The Newborn Screening market, segmented by test type, includes key categories such as biochemical tests, genetic tests, and physiological tests. Biochemical tests dominate the market, accounting for $2.83 billion in 2023, with a growth forecast to $5.19 billion by 2033. Genetic tests and physiological tests are also significant, reflecting the industry's shift towards advanced testing methodologies.
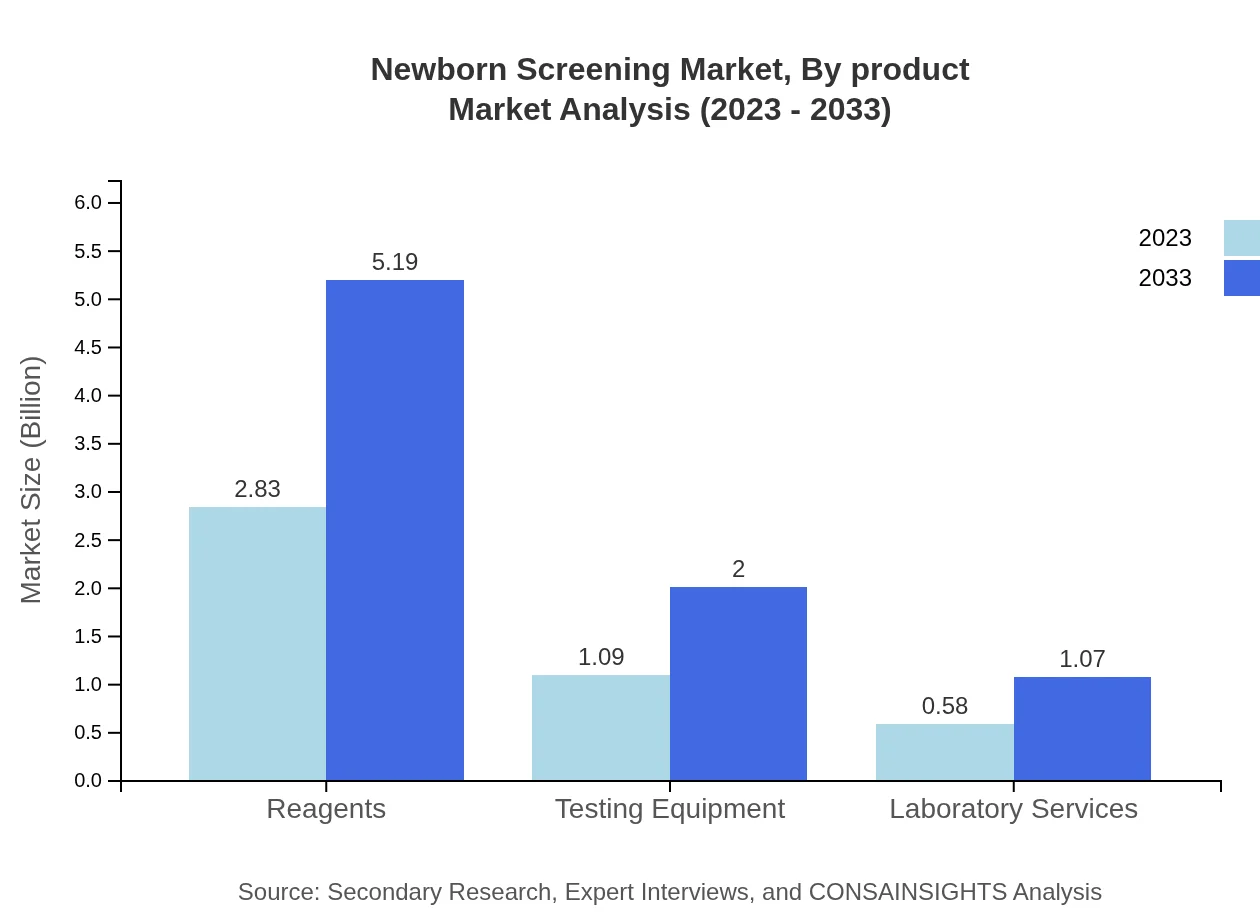
Newborn Screening Market Analysis By Product
Products used in newborn screening include reagents, testing equipment, and laboratory services. Reagents account for a substantial market share, expected to grow from $2.83 billion in 2023 to $5.19 billion in 2033, while testing equipment is projected to rise from $1.09 billion to $2.00 billion during the same period.
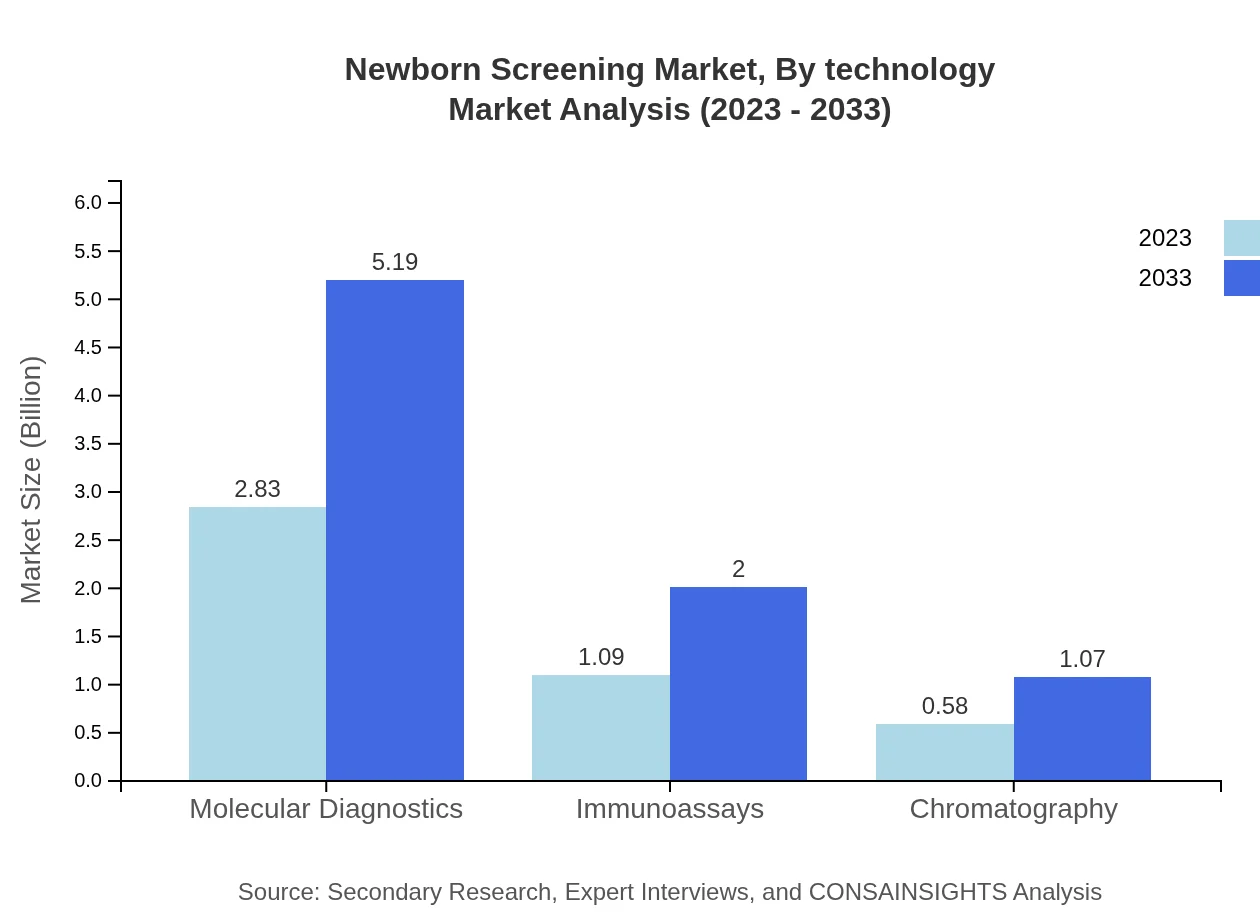
Newborn Screening Market Analysis By Technology
The technology segment of the Newborn Screening market is advancing rapidly, influenced by molecular diagnostics, immunoassays, chromatography, and other technologies. Molecular diagnostics currently leads with a projected increase from $2.83 billion in 2023 to $5.19 billion by 2033.
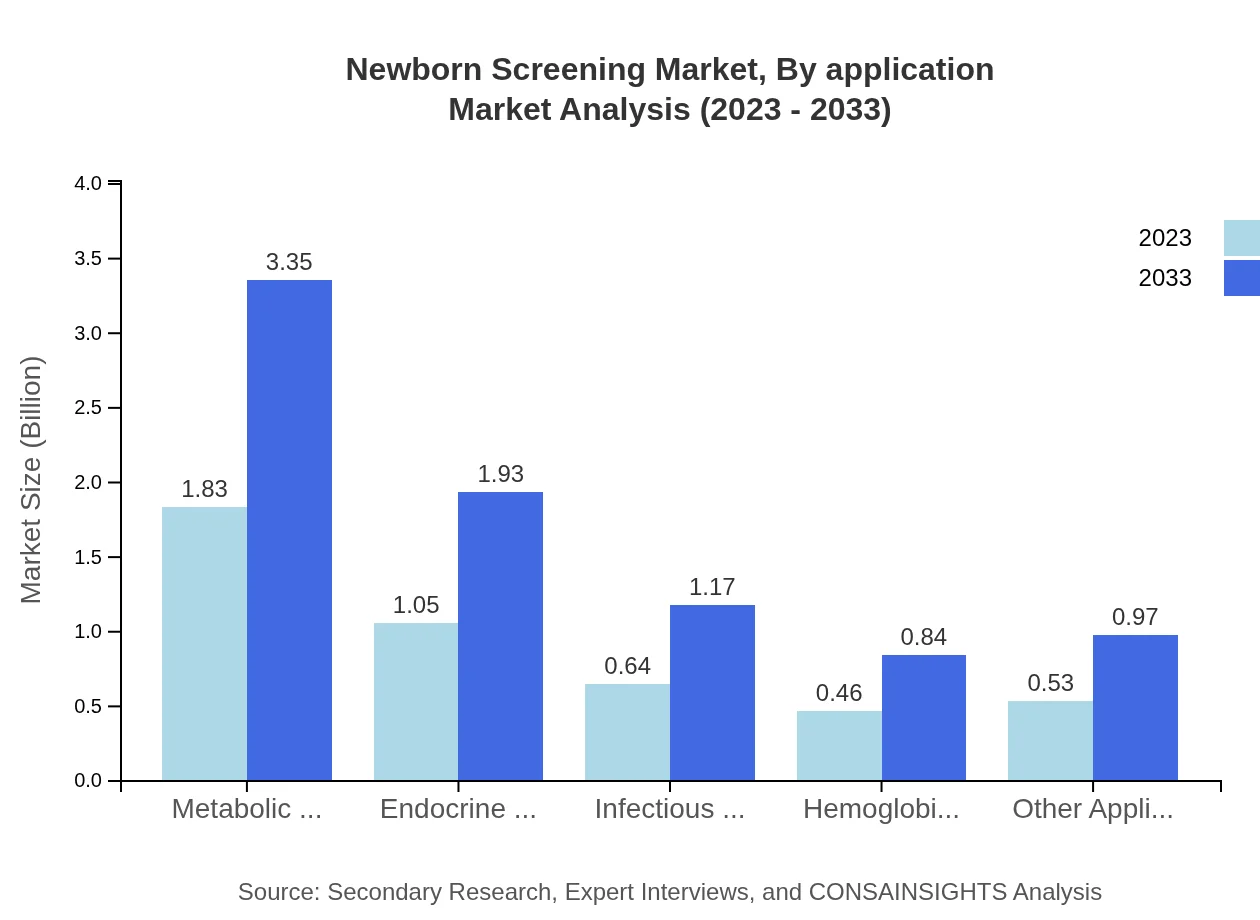
Newborn Screening Market Analysis By Application
The primary applications within the Newborn Screening market encompass metabolic disorders, endocrine disorders, infectious diseases, and more. Metabolic disorders are the largest segment growing from $1.83 billion in 2023 to $3.35 billion in 2033, boosted by increased screening initiatives and awareness.
Newborn Screening Market Trends and Future Forecast
Tell us your focus area and get a customized research report.
Global Market Leaders and Top Companies in Newborn Screening Industry
PerkinElmer, Inc.:
A global leader in newborn screening solutions, PerkinElmer provides a wide range of innovative tests and technologies to ensure the health and wellbeing of newborns.Abbott Laboratories:
Abbott is a major player in the healthcare industry, offering comprehensive newborn screening products that leverage advanced technology for effective screening.Thermo Fisher Scientific:
Thermo Fisher Scientific provides various diagnostics solutions for newborn screening, emphasizing innovation and high-quality testing.Agilent Technologies:
Agilent focuses on advanced instrumentations and technologies for genetic screening, enhancing the accuracy and efficiency of newborn health assessments.Roche Diagnostics:
Roche provides diagnostic solutions that aid in the early detection of various metabolic conditions, ensuring that newborns receive timely interventions.We're grateful to work with incredible clients.









FAQs
What is the market size of newborn Screening?
The global newborn screening market is valued at approximately $4.5 billion in 2023, with an expected CAGR of 6.1% from 2023 to 2033. This growth reflects the increasing importance of early disease detection in newborns.
What are the key market players or companies in the newborn Screening industry?
Key players in the newborn screening market include major diagnostic companies and healthcare providers that specialize in genetic testing, laboratory services, and manufacturing of screening equipment, contributing to the competitive landscape of the industry.
What are the primary factors driving the growth in the newborn screening industry?
Growth in the newborn screening industry is driven by advancements in technology, increased awareness of genetic disorders, the rising incidence of metabolic disorders, and supportive regulations promoting early detection and intervention.
Which region is the fastest Growing in the newborn screening?
The fastest-growing region in the newborn screening market is Asia Pacific, with the market projected to increase from $0.98 billion in 2023 to $1.79 billion by 2033. North America also shows significant growth potential.
Does ConsaInsights provide customized market report data for the newborn screening industry?
Yes, ConsaInsights offers customized market report data tailored to specific needs within the newborn screening industry, enabling clients to gain insights into particular areas of interest, including regional and segment analyses.
What deliverables can I expect from this newborn screening market research project?
Clients can expect comprehensive deliverables including in-depth market analysis, trends, forecasts, competitive landscape overview, and tailored insights on regional and segment performance in the newborn screening market.
What are the market trends of newborn screening?
Market trends in newborn screening include a shift towards molecular diagnostics, increased utilization of genetic tests, and the development of sophisticated laboratory services to enhance screening efficiency and accuracy.