Next Generation Antibody Therapeutics Market Report
Published Date: 31 January 2026 | Report Code: next-generation-antibody-therapeutics
Next Generation Antibody Therapeutics Market Size, Share, Industry Trends and Forecast to 2033
This report provides a comprehensive analysis of the Next Generation Antibody Therapeutics market from 2023 to 2033, covering market sizing, regional insights, technology trends, and industry leadership dynamics.
| Metric | Value |
|---|---|
| Study Period | 2023 - 2033 |
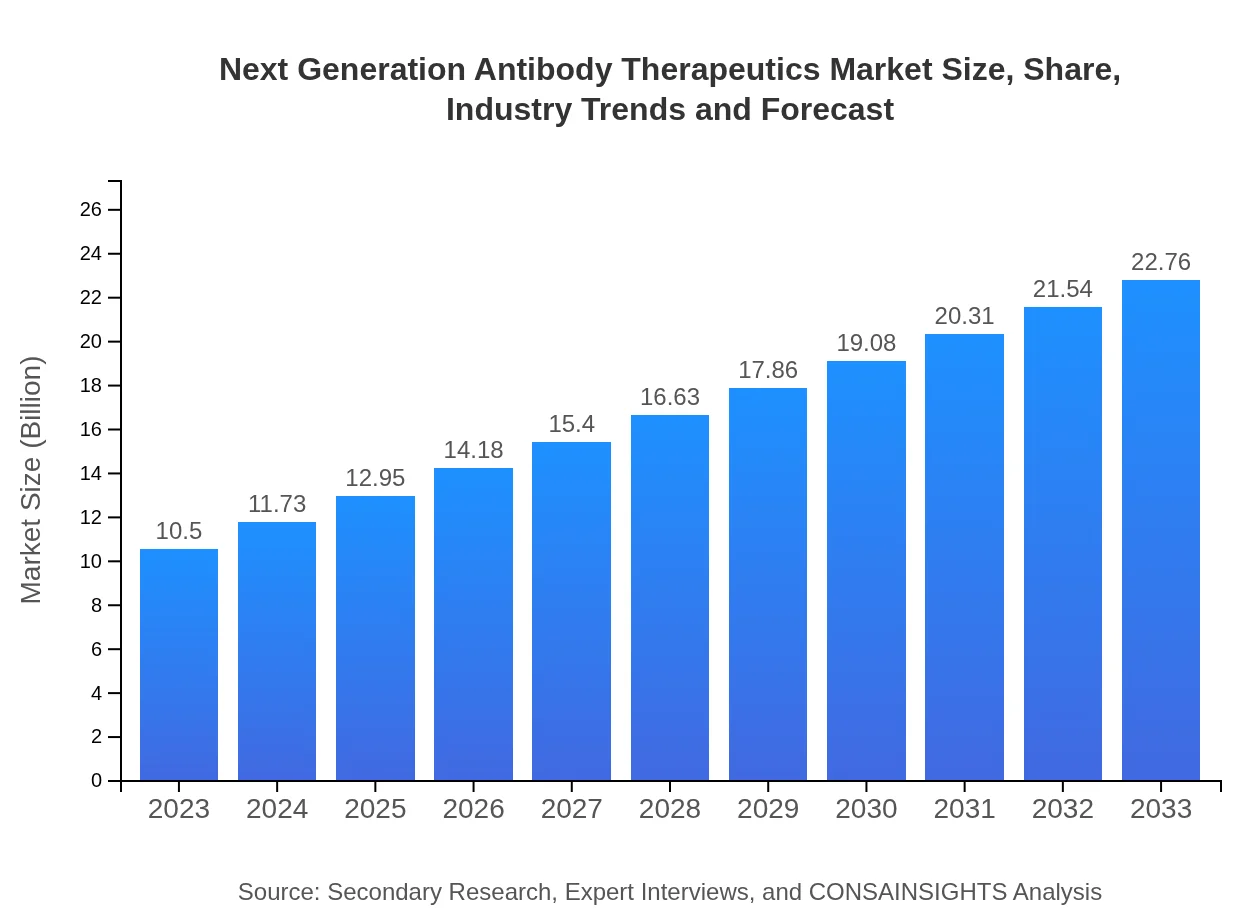
| 2023 Market Size | $10.50 Billion |
| CAGR (2023-2033) | 7.8% |
| 2033 Market Size | $22.76 Billion |
| Top Companies | Roche, Amgen, AbbVie, Bristol-Myers Squibb, Johnson & Johnson |
| Last Modified Date | 31 January 2026 |
Next Generation Antibody Therapeutics Market Overview
Customize Next Generation Antibody Therapeutics Market Report market research report
- ✔ Get in-depth analysis of Next Generation Antibody Therapeutics market size, growth, and forecasts.
- ✔ Understand Next Generation Antibody Therapeutics's regional dynamics and industry-specific trends.
- ✔ Identify potential applications, end-user demand, and growth segments in Next Generation Antibody Therapeutics
What is the Market Size & CAGR of Next Generation Antibody Therapeutics market in 2023?
Next Generation Antibody Therapeutics Industry Analysis
Next Generation Antibody Therapeutics Market Segmentation and Scope
Tell us your focus area and get a customized research report.
Next Generation Antibody Therapeutics Market Analysis Report by Region
Europe Next Generation Antibody Therapeutics Market Report:
Europe's market is forecasted to grow from $3.56 billion in 2023 to $7.72 billion by 2033, driven by substantial investments in healthcare infrastructure and increasing demand for advanced therapeutics from both public and private sectors. Countries like Germany and the UK are spearheading this growth.Asia Pacific Next Generation Antibody Therapeutics Market Report:
In the Asia Pacific region, the market for Next Generation Antibody Therapeutics is forecasted to reach $3.94 billion by 2033, up from $1.82 billion in 2023. Factors driving growth include increasing healthcare investments, a rising patient population, and the expanding biotechnology sector across countries like China and India.North America Next Generation Antibody Therapeutics Market Report:
North America continues to lead the market, with a projected increase from $3.60 billion in 2023 to $7.81 billion in 2033. This region benefits from advanced healthcare infrastructure, significant R&D investments by pharmaceutical companies, and a strong regulatory framework that supports rapid drug approval processes.South America Next Generation Antibody Therapeutics Market Report:
In South America, the market is poised to grow from $0.75 billion in 2023 to $1.62 billion by 2033. The growth in this region is attributed to rising healthcare expenditures and an increasing prevalence of chronic diseases that necessitate innovative therapeutic solutions.Middle East & Africa Next Generation Antibody Therapeutics Market Report:
The Middle East and Africa region is expected to expand from $0.77 billion in 2023 to $1.67 billion by 2033. The growth is propelled by increasing access to healthcare facilities and innovative therapies, alongside governmental initiatives focusing on healthcare improvements.Tell us your focus area and get a customized research report.
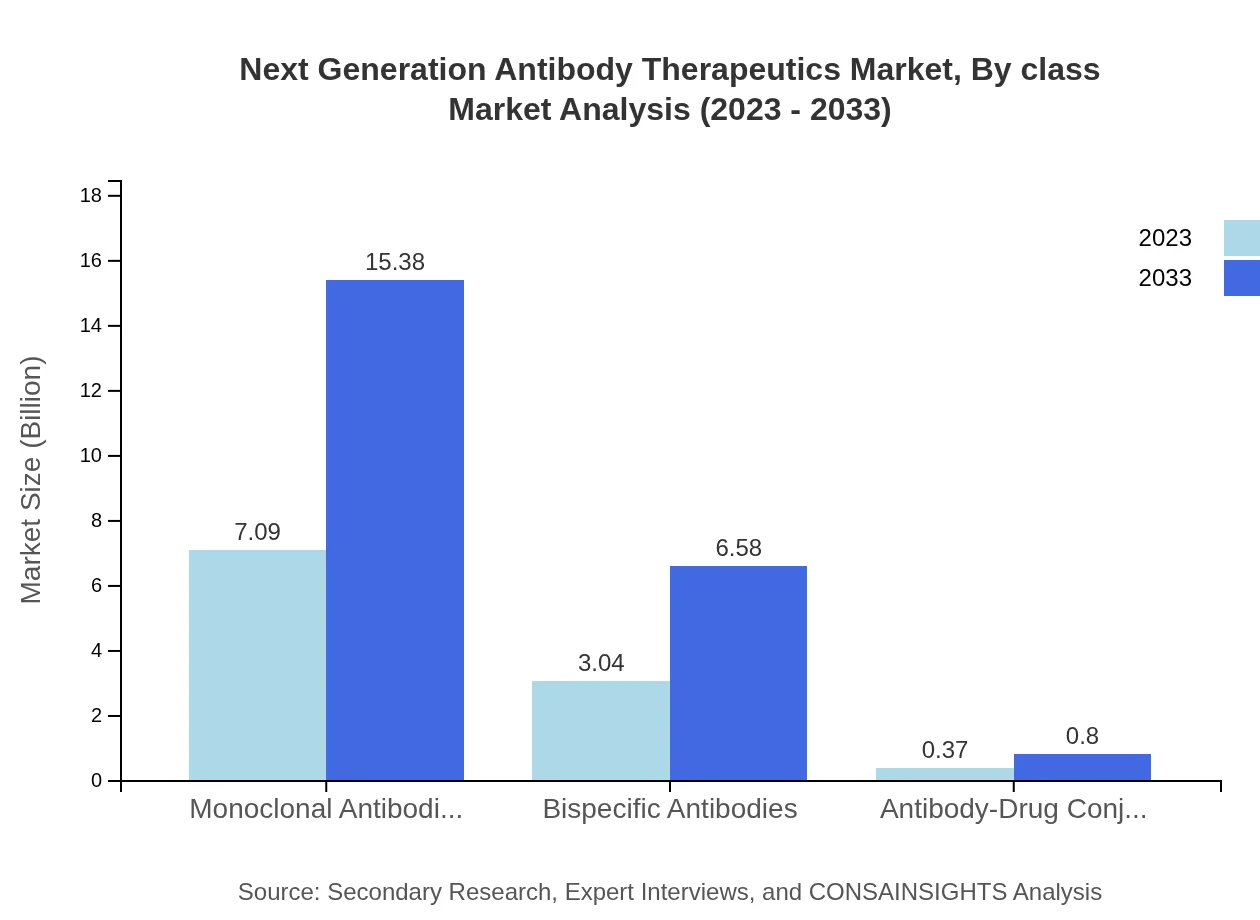
Next Generation Antibody Therapeutics Market Analysis By Class
The classification of antibody therapeutics indicates a significant presence of monoclonal antibodies in the marketplace, valued at about $7.09 billion in 2023 and expected to reach $15.38 billion by 2033. Monoclonal antibodies capture approximately 67.56% of the market share, retained through their critical role in oncology therapies. Bispecific antibodies are also making headway due to their ability to target multiple antigens simultaneously, with a market size forecasted from $3.04 billion to $6.58 billion over the same period, holding a significant 28.91% market share.
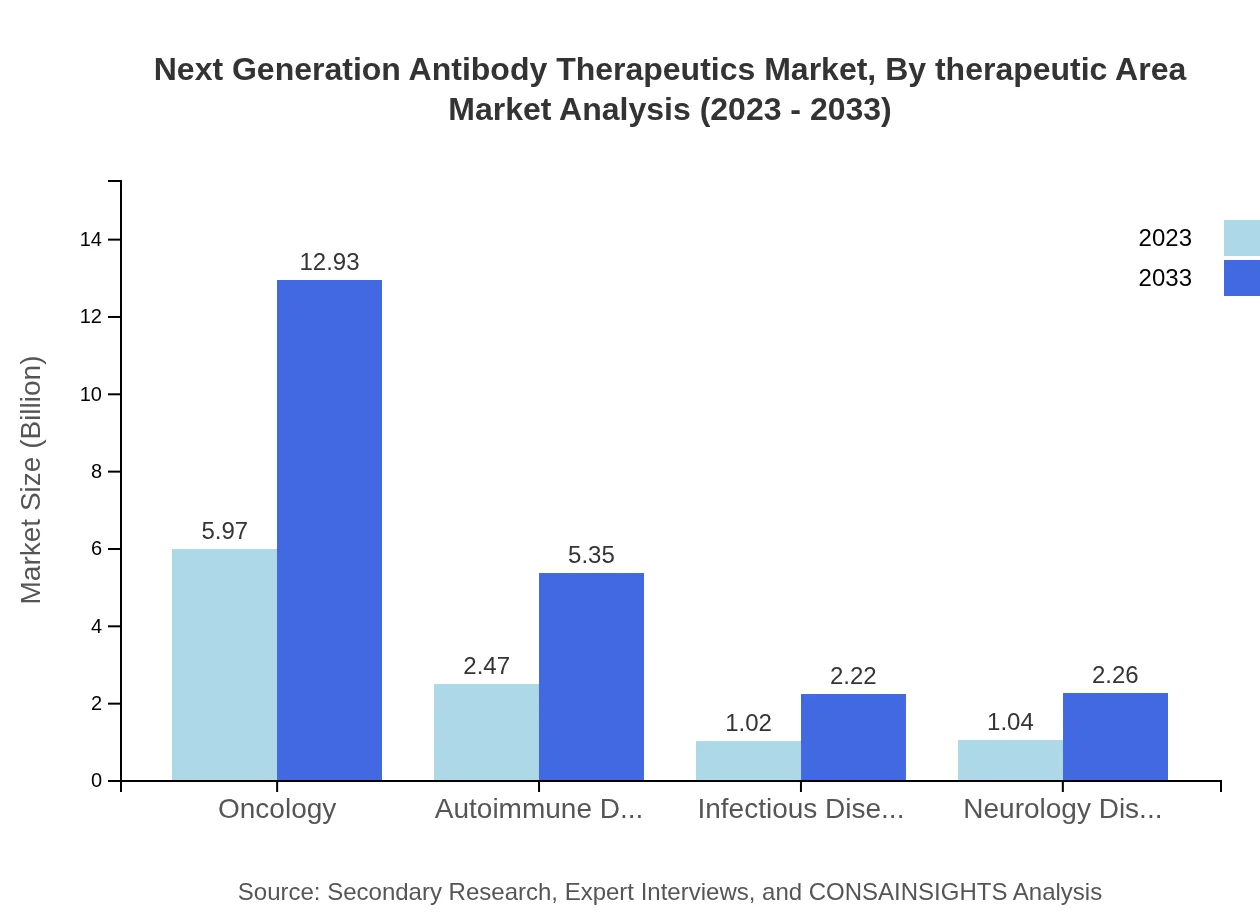
Next Generation Antibody Therapeutics Market Analysis By Therapeutic Area
Oncology remains the leading therapeutic area for next-generation antibody therapeutics, projected to grow from $5.97 billion in 2023 to $12.93 billion by 2033, maintaining a 56.82% market share. Autoimmune disorder therapeutics will follow, increasing from $2.47 billion to $5.35 billion, while infectious disease therapeutics, although demonstrating slower growth, will also gain traction, projected to rise to $2.22 billion.
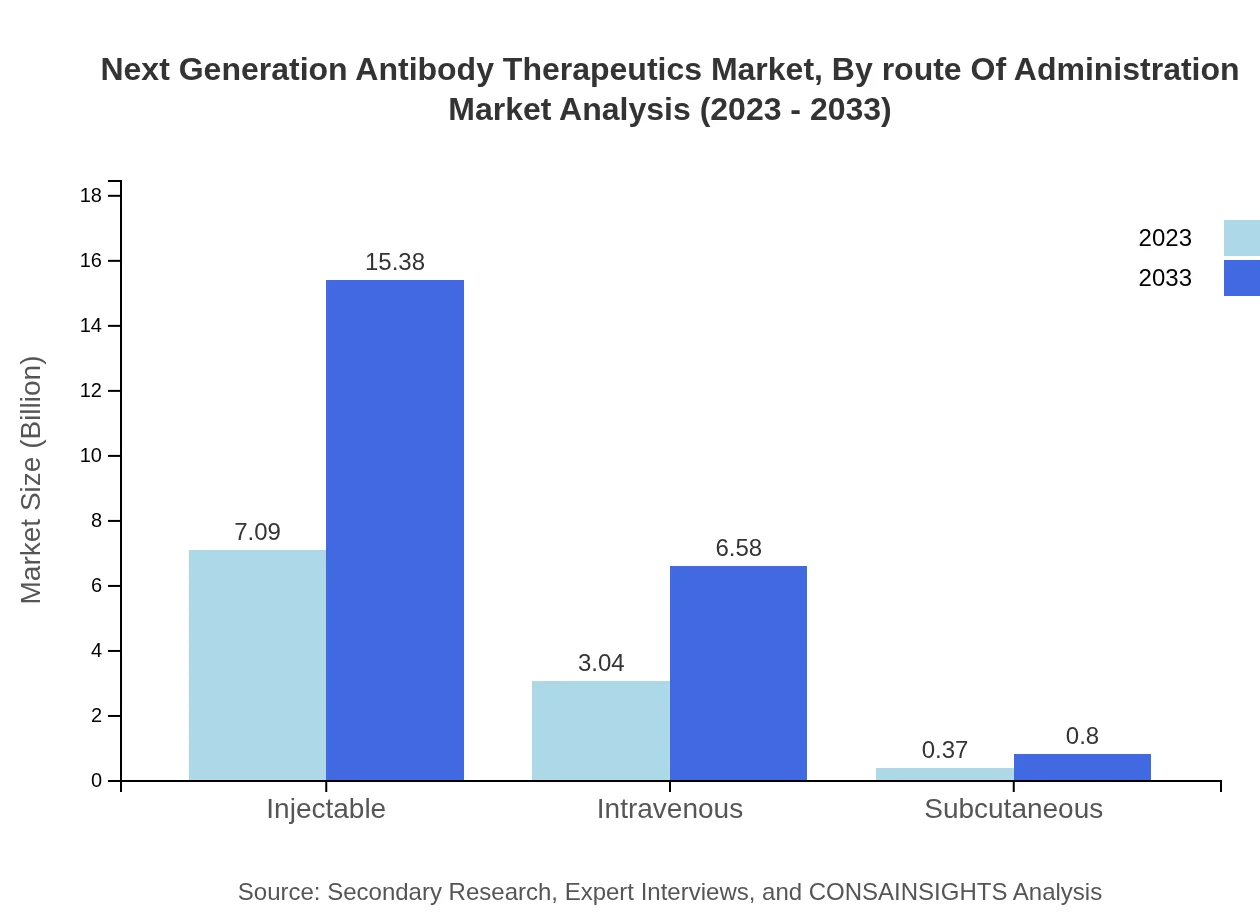
Next Generation Antibody Therapeutics Market Analysis By Route Of Administration
The routes of administration for next-generation antibodies primarily include injectable (estimated to grow from $7.09 billion to $15.38 billion), intravenous (expected to expand from $3.04 billion to $6.58 billion), and subcutaneous routes (forecasted growth from $0.37 billion to $0.80 billion). Injectable forms dominate due to their adoption in clinical settings, whilst intravenous methods remain significant for high-volume therapies.
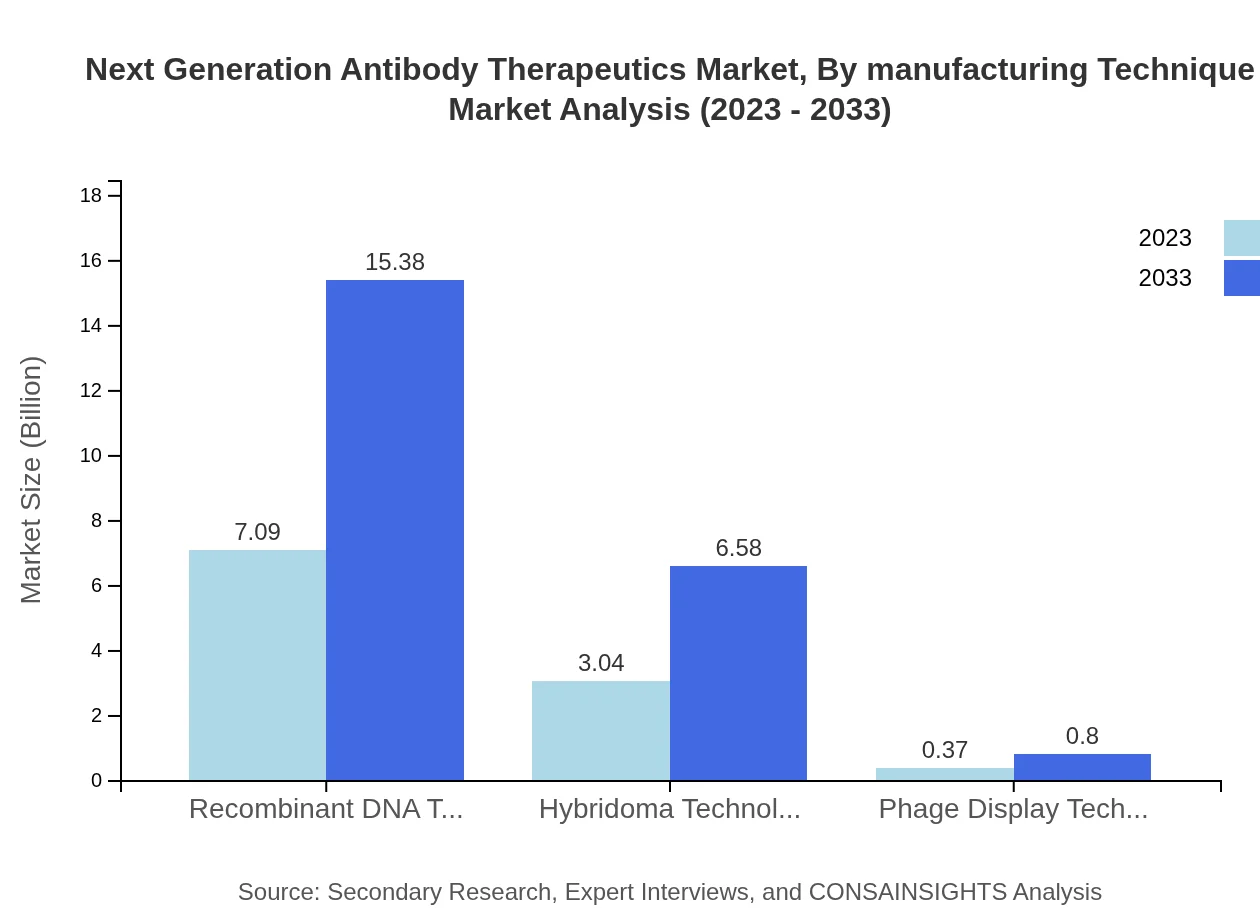
Next Generation Antibody Therapeutics Market Analysis By Manufacturing Technique
Manufacturing techniques aim to optimize yield and efficiency in producing antibody drugs. Recombinant DNA technology holds about 67.56% market share, expected to grow to 15.38 billion by 2033 as the dominant tech across the market. Hybridoma technology, while older, still plays a crucial role in some products, demonstrating stability in its share and growth from 3.04 billion to 6.58 billion.
Next Generation Antibody Therapeutics Market Analysis By End User
The end-user segment comprises pharmaceutical companies, biotechnology firms, research institutes, and hospitals/clinics. Pharmaceutical companies dominate, increasing their market share from $5.97 billion in 2023 to $12.93 billion by 2033. Biotechnology firms also contribute significantly, growing from $2.47 billion to $5.35 billion, emphasizing the collaborative nature of modern drug development.
Next Generation Antibody Therapeutics Market Trends and Future Forecast
Tell us your focus area and get a customized research report.
Global Market Leaders and Top Companies in Next Generation Antibody Therapeutics Industry
Roche:
A pioneer in the field of biotechnology, Roche is a leader in developing monoclonal antibodies, including Herceptin and Avastin that have significantly changed cancer therapy standards.Amgen:
Known for its innovative approach to monoclonal antibodies and biotechnology, Amgen’s therapies such as Enbrel have transformed the treatment landscape for autoimmune disorders.AbbVie:
AbbVie specializes in biologic therapies and has a robust portfolio of antibody therapeutics including Humira, driving substantial revenues in the antibody therapeutic market.Bristol-Myers Squibb:
Bristol-Myers Squibb focuses on immunotherapy and has engaged in pioneering studies on bispecific antibodies and ADCs, significantly impacting oncology treatments.Johnson & Johnson:
This conglomerate has a diversified portfolio, including a strong line of antibody therapies across oncological and autoimmune indications.We're grateful to work with incredible clients.









FAQs
What is the market size of Next Generation Antibody Therapeutics?
The global market for Next Generation Antibody Therapeutics is projected to reach approximately $10.5 billion by 2033, growing at a CAGR of 7.8%. This growth indicates strong demand and advancements in antibody therapies.
What are the key market players or companies in the Next Generation Antibody Therapeutics industry?
Key players in this industry include leading pharmaceutical companies and biotechnology firms that specialize in monoclonal antibodies, bispecific antibodies, and antibody-drug conjugates, continually innovating to improve clinical outcomes.
What are the primary factors driving the growth in the Next Generation Antibody Therapeutics industry?
Factors include increasing prevalence of cancer and autoimmune diseases, advancements in biotechnology, expanded research funding, and greater acceptance of targeted therapies, contributing to a robust growth trajectory.
Which region is the fastest Growing in the Next Generation Antibody Therapeutics?
The fastest-growing region is expected to be Europe, with market sizes projected to increase from $3.56 billion in 2023 to $7.72 billion in 2033, showcasing significant investment and demand in biotech research.
Does ConsaInsights provide customized market report data for the Next Generation Antibody Therapeutics industry?
Yes, ConsaInsights offers customized market report data tailored to specific needs, providing in-depth analysis and insights that cater to individual client requirements in the Next Generation Antibody Therapeutics market.
What deliverables can I expect from this Next Generation Antibody Therapeutics market research project?
Expect comprehensive market analysis, segmented data insights, trends identification, competitive landscape assessments, and tailored recommendations to support strategic decisions and investments.
What are the market trends of Next Generation Antibody Therapeutics?
Current trends include increasing focus on personalized medicine, integration of artificial intelligence in drug discovery, growth of combination therapies, and an emphasis on reducing production costs while enhancing therapeutic efficacy.