Nitinol Medical Devices Market Report
Published Date: 31 January 2026 | Report Code: nitinol-medical-devices
Nitinol Medical Devices Market Size, Share, Industry Trends and Forecast to 2033
This report provides a comprehensive analysis of the Nitinol Medical Devices market, covering insights on market size, growth trends, segmentation, regional analysis, and industry leaders within the forecast period from 2023 to 2033.
| Metric | Value |
|---|---|
| Study Period | 2023 - 2033 |
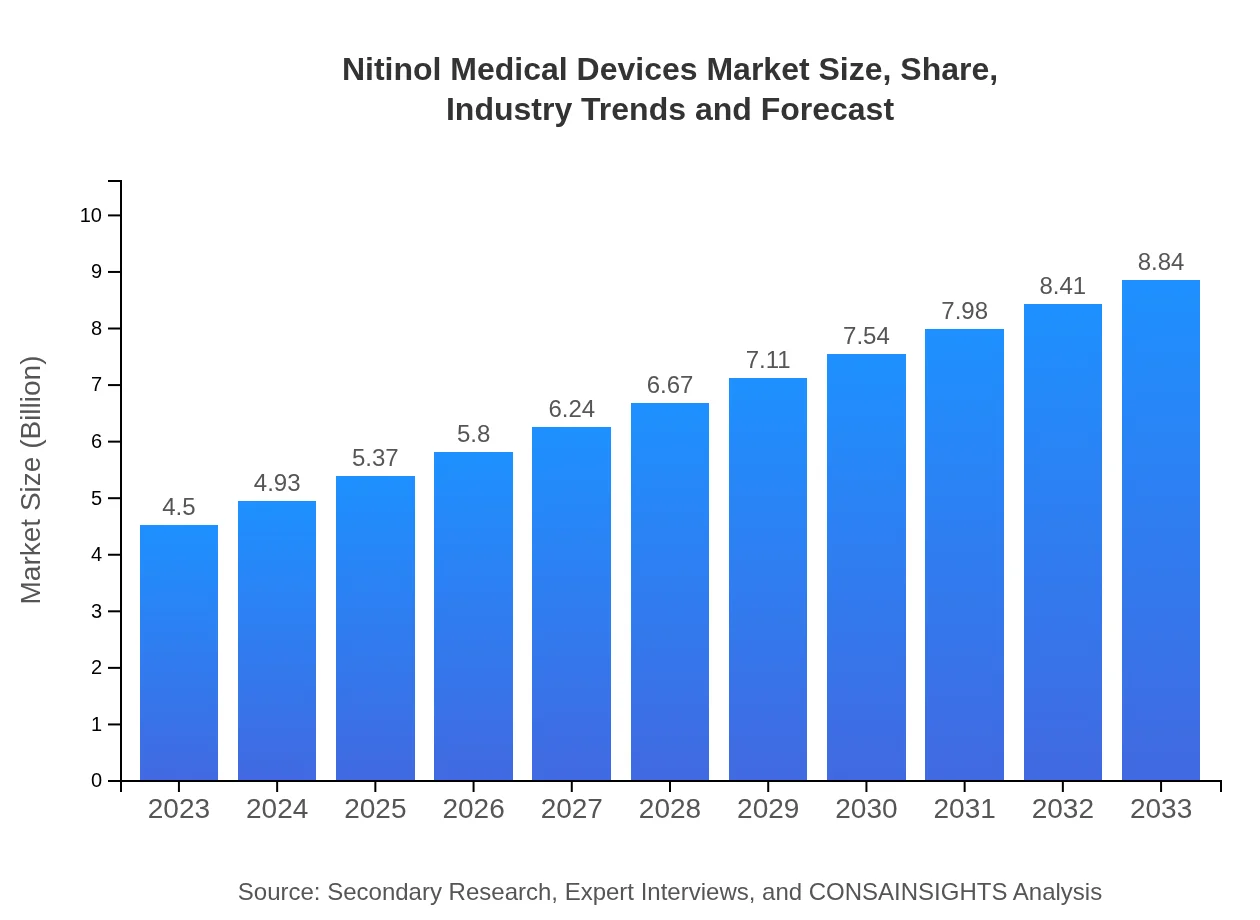
| 2023 Market Size | $4.50 Billion |
| CAGR (2023-2033) | 6.8% |
| 2033 Market Size | $8.84 Billion |
| Top Companies | Medtronic , Abbott Laboratories, Boston Scientific, Cook Medical |
| Last Modified Date | 31 January 2026 |
Nitinol Medical Devices Market Overview
Customize Nitinol Medical Devices Market Report market research report
- ✔ Get in-depth analysis of Nitinol Medical Devices market size, growth, and forecasts.
- ✔ Understand Nitinol Medical Devices's regional dynamics and industry-specific trends.
- ✔ Identify potential applications, end-user demand, and growth segments in Nitinol Medical Devices
What is the Market Size & CAGR of Nitinol Medical Devices market in 2023?
Nitinol Medical Devices Industry Analysis
Nitinol Medical Devices Market Segmentation and Scope
Tell us your focus area and get a customized research report.
Nitinol Medical Devices Market Analysis Report by Region
Europe Nitinol Medical Devices Market Report:
The European market stands at $1.59 billion in 2023, anticipated to surge to $3.12 billion by 2033, propelled by favorable regulatory conditions and increasing investments in healthcare.Asia Pacific Nitinol Medical Devices Market Report:
In the Asia Pacific region, the Nitinol Medical Devices market is valued at $0.78 billion in 2023, with expectations of reaching $1.53 billion by 2033, driven by increasing healthcare infrastructure and rising surgical procedures.North America Nitinol Medical Devices Market Report:
North America is a leading market with a size of $1.50 billion in 2023, projected to attain $2.95 billion by 2033, fueled by advanced medical technology and high demand for innovative medical devices.South America Nitinol Medical Devices Market Report:
The South American market is forecast to grow from $0.02 billion in 2023 to $0.04 billion by 2033, albeit at a slower rate due to limited healthcare funding and adoption rates.Middle East & Africa Nitinol Medical Devices Market Report:
The Middle East and Africa market is valued at $0.61 billion in 2023, projected to grow to $1.20 billion by 2033, spurred by improvements in medical technology and increased healthcare initiatives.Tell us your focus area and get a customized research report.
Nitinol Medical Devices Market Analysis By Product
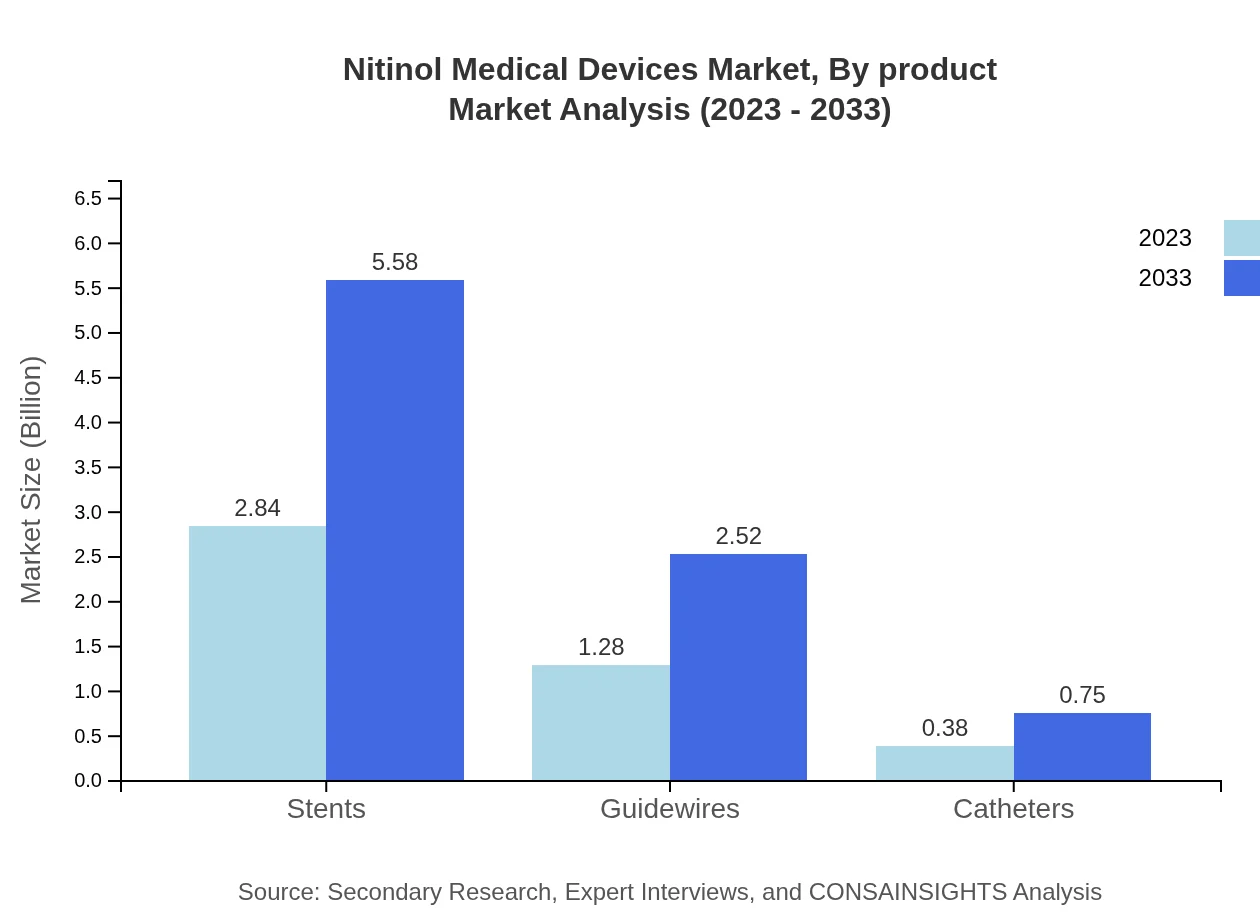
In 2023, the Nitinol Medical Devices segmented by product demonstrated a robust performance. The standard design segment leads with a market size of $3.81 billion, projected to rise to $7.49 billion by 2033. Custom design contributes with $0.69 billion in 2023 and is expected to reach $1.36 billion. Stents, a significant contributor, account for $2.84 billion in 2023, expected to grow to $5.58 billion. Guidewires hold a market size of $1.28 billion, anticipated to reach $2.52 billion, while catheters start at $0.38 billion and can reach $0.75 billion by 2033.
Nitinol Medical Devices Market Analysis By Application
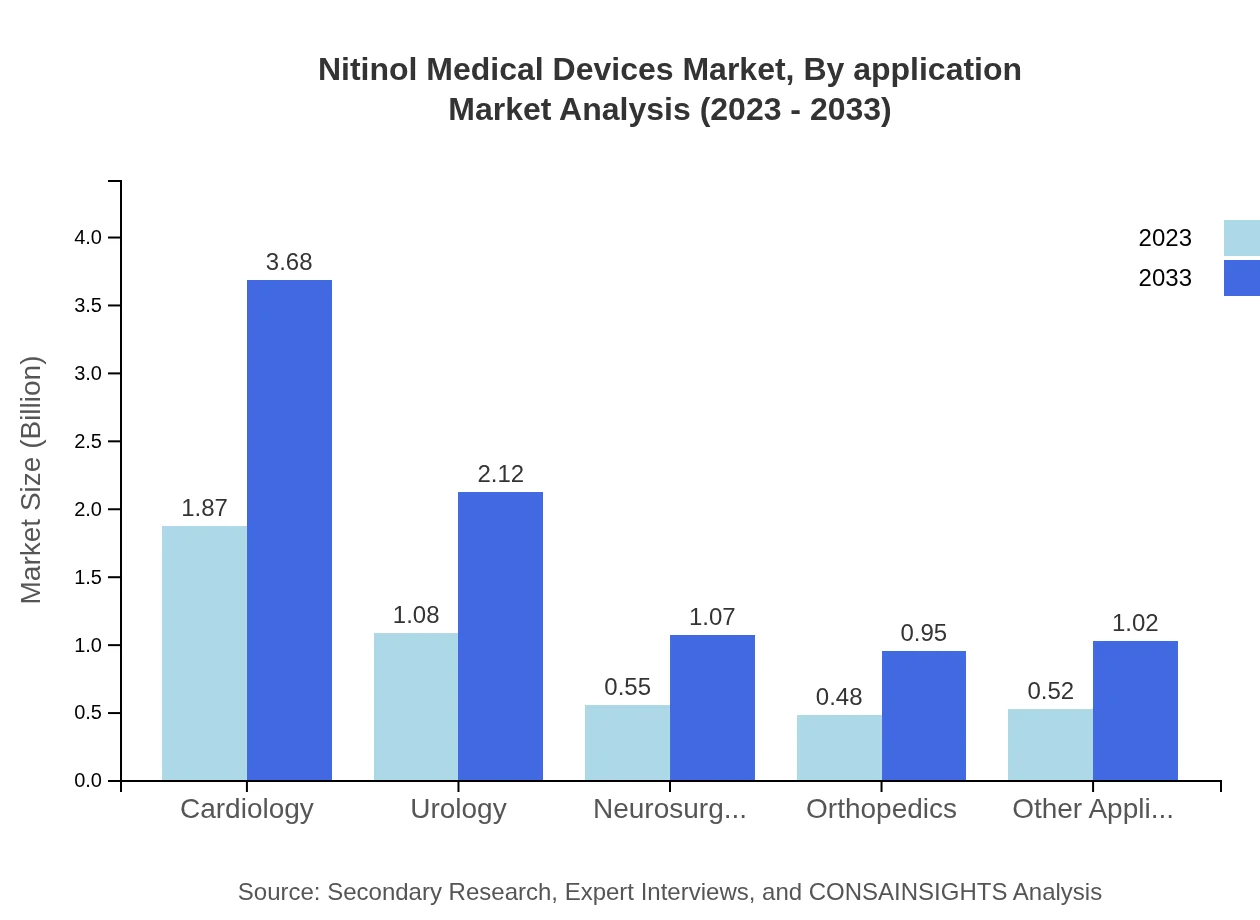
Applications of Nitinol devices are mainly in cardiology, urology, and neurosurgery, with cardiology leading at $1.87 billion in 2023 and projected to grow to $3.68 billion. Urology has an initial market size of $1.08 billion, estimated to climb to $2.12 billion while neurosurgery starts at $0.55 billion, advancing to $1.07 billion. A smaller share, other applications see growth from $0.52 billion to $1.02 billion over the decade.
Nitinol Medical Devices Market Analysis By End User
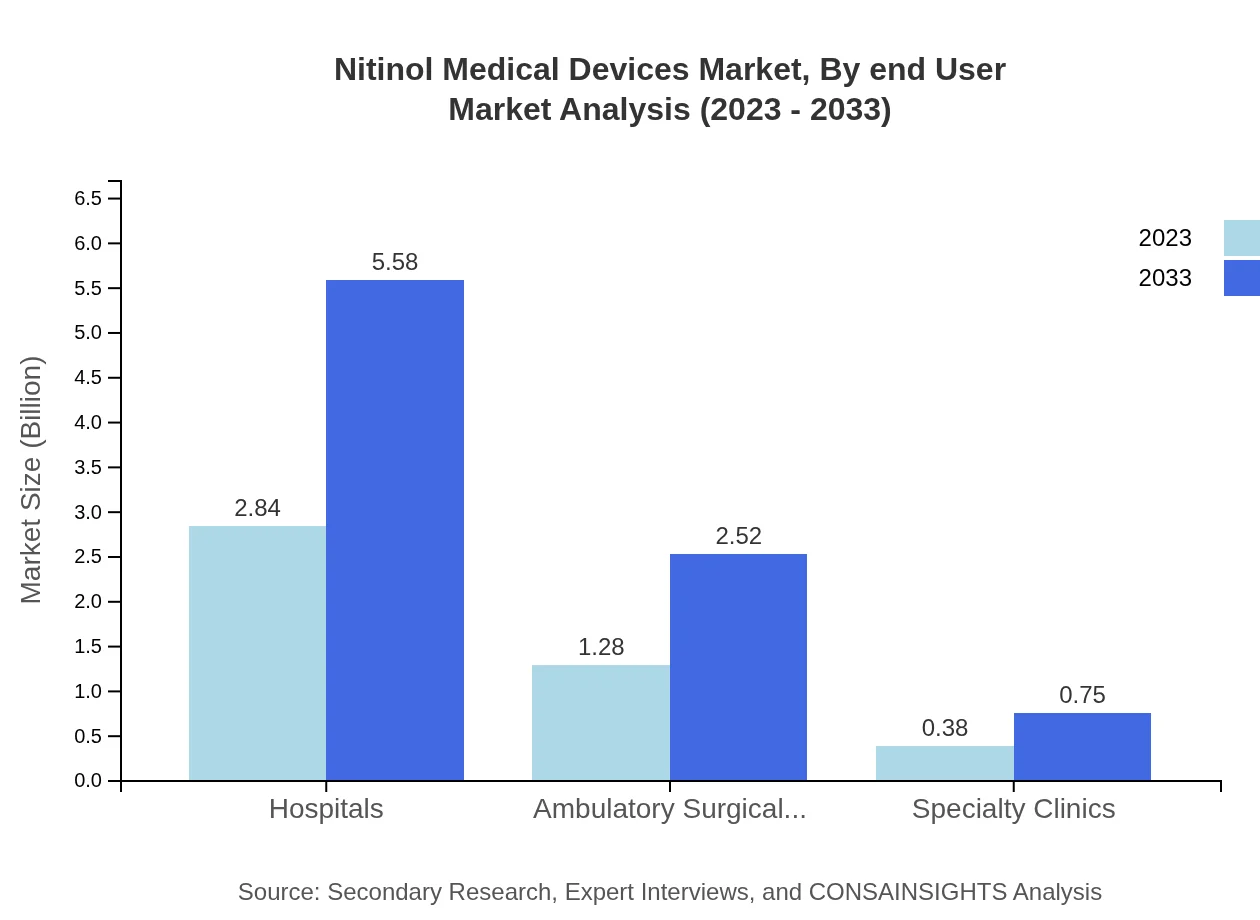
The end-user analysis reveals hospitals as the primary consumers, starting at $2.84 billion, growing to $5.58 billion in 2033, while ambulatory surgical centers begin at $1.28 billion, with projected growth to $2.52 billion. Specialty clinics represent a smaller fraction, starting at $0.38 billion, anticipated to double by 2033.
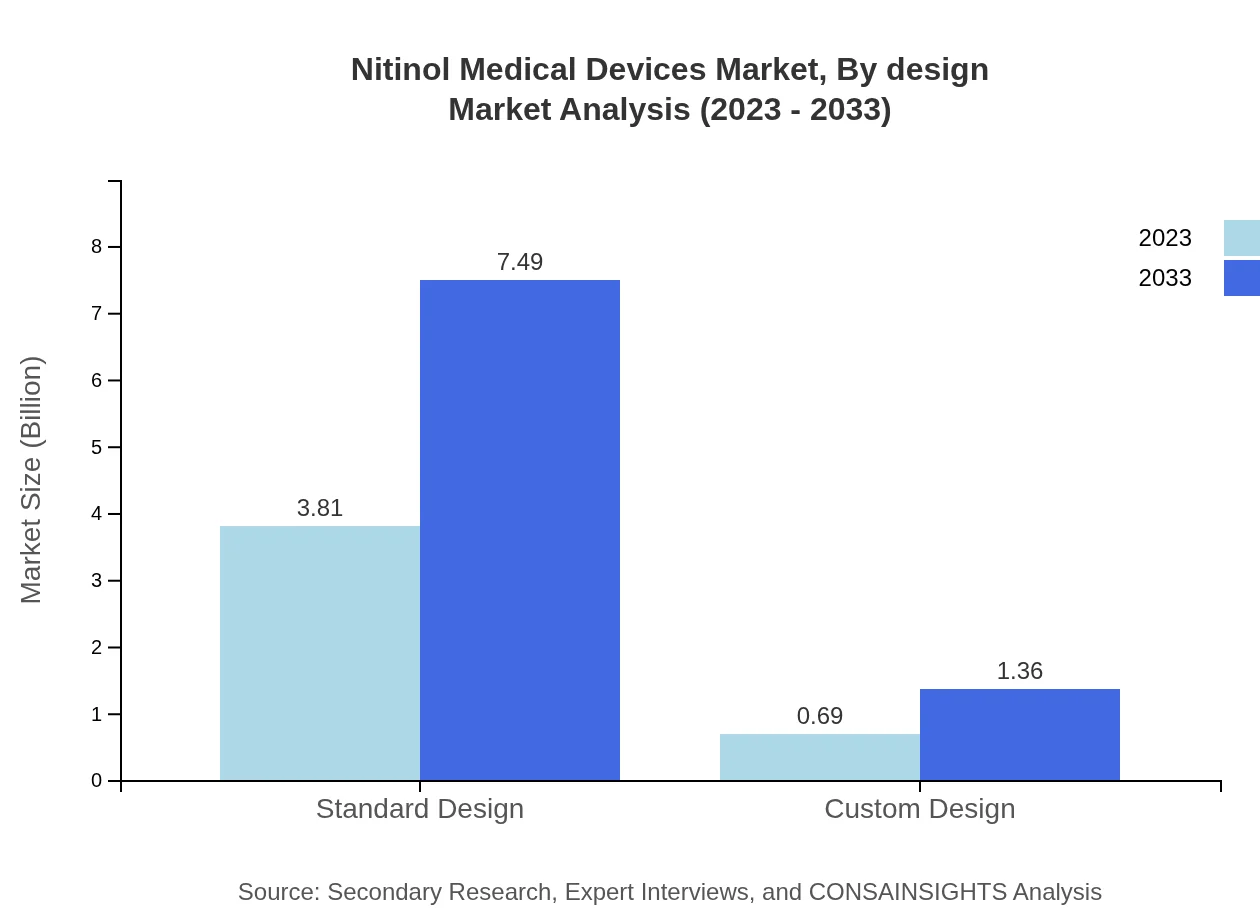
Nitinol Medical Devices Market Analysis By Design
Products designed using Nitinol, such as shape memory alloy and superelastic processing, show significant potential. The shape memory alloy processing market is valued at $3.81 billion in 2023 and is expected to grow to $7.49 billion, while superelastic processing remains at $0.69 billion, with a forecasted rise to $1.36 billion.
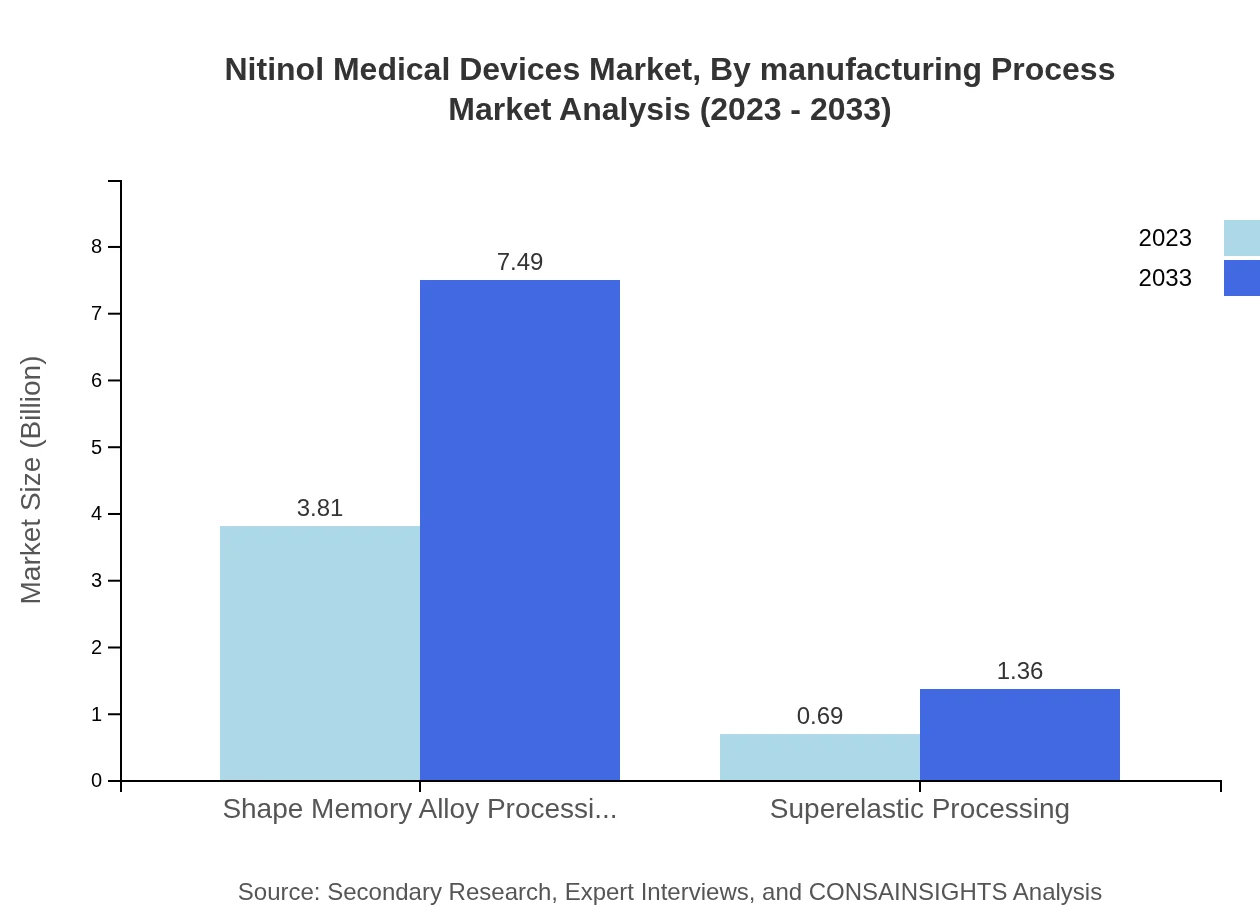
Nitinol Medical Devices Market Analysis By Manufacturing Process
Manufacturing processes significantly define the market dynamics of Nitinol devices. Technologies such as standard and custom designs dominate the landscape, with various applications across medical fields driving innovations and efficiency improvements in production.
Nitinol Medical Devices Market Trends and Future Forecast
Tell us your focus area and get a customized research report.
Global Market Leaders and Top Companies in Nitinol Medical Devices Industry
Medtronic :
A pioneer in medical technology, Medtronic produces a range of Nitinol devices, improving patient outcomes through innovation and advanced solutions in interventional procedures.Abbott Laboratories:
Abbott plays a crucial role in Nitinol device markets, focusing on stents and guidewires, with a dedication to enhancing minimally invasive techniques across various applications.Boston Scientific:
Boston Scientific is renowned for its development of Nitinol-based products, particularly in cardiology and urology, emphasizing innovation and technology to address patient needs.Cook Medical:
Cook Medical specializes in customized Nitinol device solutions, encouraging advancements in therapeutic procedures within the healthcare sector.We're grateful to work with incredible clients.









FAQs
What is the market size of Nitinol Medical Devices?
The Nitinol Medical Devices market is valued at $4.5 billion in 2023, with a CAGR of 6.8%. Projections indicate potential growth by 2033, supported by advancements and increased adoption in various medical fields.
What are the key market players or companies in the Nitinol Medical Devices industry?
Key players in the Nitinol Medical Devices market include major corporations specializing in medical device technology, known for their innovative products and significant market share, which can influence growth trends and competitive dynamics.
What are the primary factors driving the growth in the Nitinol Medical Devices industry?
Growth factors include increasing demand for minimally invasive surgeries, technological advancements in medical devices, and rising incidences of chronic diseases that necessitate innovative treatment solutions, propelling market expansion.
Which region is the fastest Growing in the Nitinol Medical Devices market?
Europe is the fastest-growing region, expected to grow from $1.59 billion in 2023 to $3.12 billion by 2033. This growth is attributed to strong healthcare infrastructure and rising tech innovations.
Does ConsaInsights provide customized market report data for the Nitinol Medical Devices industry?
Yes, ConsaInsights offers customized market report data for the Nitinol Medical Devices industry, tailoring insights to meet specific needs and providing detailed analyses based on client requirements.
What deliverables can I expect from this Nitinol Medical Devices market research project?
Deliverables include comprehensive reports detailing market size, trends, forecasts, competitive analysis, segment data, and regional insights, ensuring clients have the necessary information for informed decisions.
What are the market trends of Nitinol Medical Devices?
Market trends indicate a focus on advanced applications in endovascular devices and growth in customization of designs, with standard designs currently dominating the market. This emphasizes innovation in patient-specific treatment options.