Non Invasive Intracranial Pressure Monitoring Devices Market Report
Published Date: 31 January 2026 | Report Code: non-invasive-intracranial-pressure-monitoring-devices
Non Invasive Intracranial Pressure Monitoring Devices Market Size, Share, Industry Trends and Forecast to 2033
This report provides a comprehensive analysis of the Non Invasive Intracranial Pressure Monitoring Devices market from 2023 to 2033. Insights include market size, growth forecasts, trends, technological advancements, and key players shaping the industry landscape.
| Metric | Value |
|---|---|
| Study Period | 2023 - 2033 |
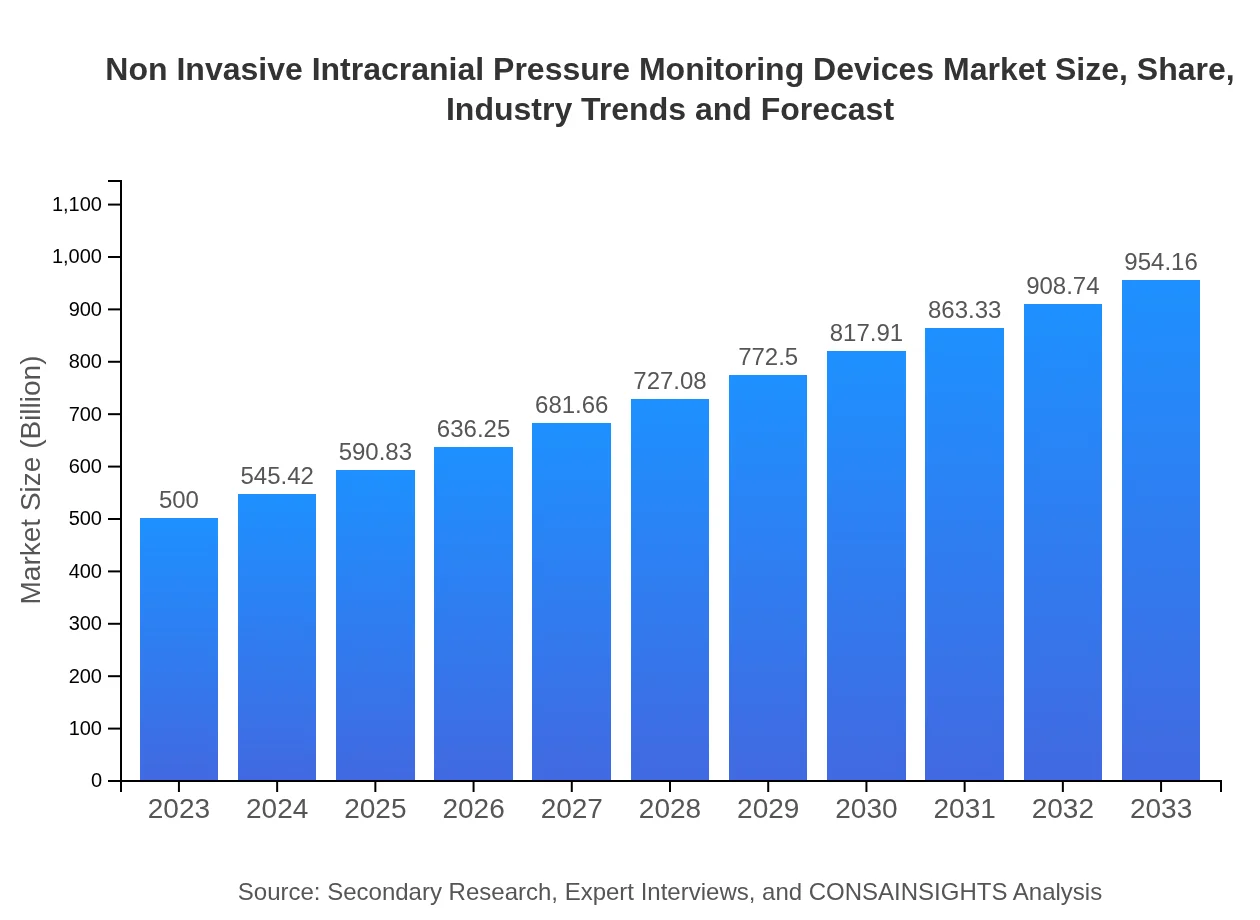
| 2023 Market Size | $500.00 Million |
| CAGR (2023-2033) | 6.5% |
| 2033 Market Size | $954.16 Million |
| Top Companies | Natus Medical Incorporated, Medtronic , Cerebrotech Medical Systems, Inc., Raumedic AG |
| Last Modified Date | 31 January 2026 |
Non Invasive Intracranial Pressure Monitoring Devices Market Overview
Customize Non Invasive Intracranial Pressure Monitoring Devices Market Report market research report
- ✔ Get in-depth analysis of Non Invasive Intracranial Pressure Monitoring Devices market size, growth, and forecasts.
- ✔ Understand Non Invasive Intracranial Pressure Monitoring Devices's regional dynamics and industry-specific trends.
- ✔ Identify potential applications, end-user demand, and growth segments in Non Invasive Intracranial Pressure Monitoring Devices
What is the Market Size & CAGR of Non Invasive Intracranial Pressure Monitoring Devices market in 2023?
Non Invasive Intracranial Pressure Monitoring Devices Industry Analysis
Non Invasive Intracranial Pressure Monitoring Devices Market Segmentation and Scope
Tell us your focus area and get a customized research report.
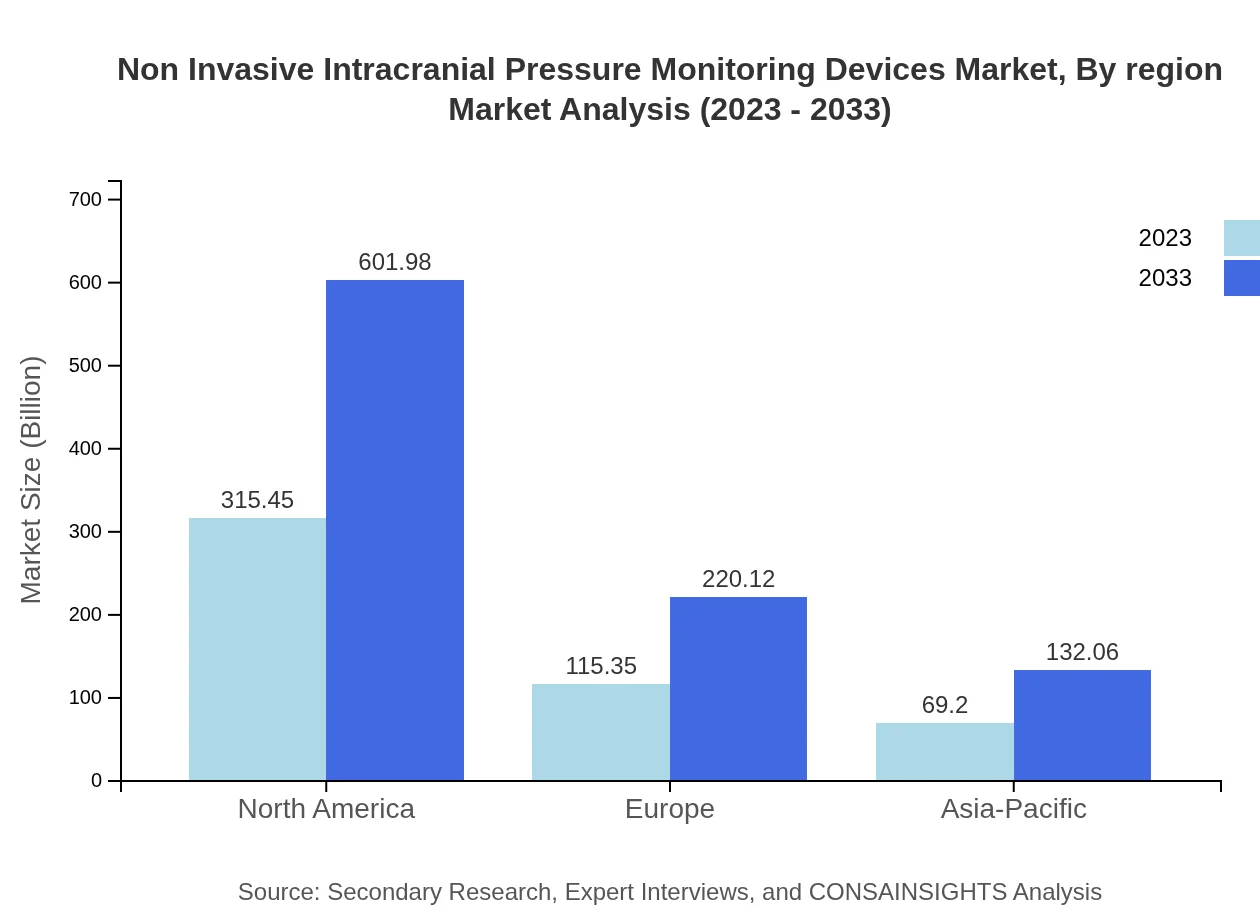
Non Invasive Intracranial Pressure Monitoring Devices Market Analysis Report by Region
Europe Non Invasive Intracranial Pressure Monitoring Devices Market Report:
Europe reflects a robust market, projected to expand from $152.25 million in 2023 to $290.54 million by 2033. The presence of advanced healthcare systems, stringent regulatory frameworks, and rising investments in neurocritical care technologies significantly contribute to its growth, alongside the aging population which demands better medical services.Asia Pacific Non Invasive Intracranial Pressure Monitoring Devices Market Report:
In the Asia Pacific region, the Non Invasive ICP Monitoring Devices market is anticipated to grow from $88.15 million in 2023 to around $168.22 million by 2033. The growth is fueled by enhancing healthcare infrastructure and a growing emphasis on advanced medical technologies in countries like China, India, and Japan. Additionally, an increasing elderly population is driving demand for diagnostics and monitoring devices.North America Non Invasive Intracranial Pressure Monitoring Devices Market Report:
The North American market, with a current value of $184.90 million in 2023, is projected to grow to approximately $352.85 million by 2033. The region is a leader in medical technology innovations, with continuous investments in R&D and healthcare infrastructure. The increasing prevalence of neurological conditions reinforces the strong demand for safe and effective monitoring devices.South America Non Invasive Intracranial Pressure Monitoring Devices Market Report:
The South American market is expected to experience significant growth, advancing from $38.20 million in 2023 to $72.90 million by 2033. This growth can be attributed to the rising healthcare expenditure and the proliferation of healthcare facilities in Brazil and Argentina, coupled with increasing public awareness of neurological disorders.Middle East & Africa Non Invasive Intracranial Pressure Monitoring Devices Market Report:
The Middle East and Africa market is anticipated to grow from $36.50 million in 2023 to approximately $69.65 million by 2033. The region is witnessing improvements in healthcare access and the introduction of state-of-the-art medical devices. Countries such as the UAE and South Africa are particularly focusing on enhancing their healthcare systems, thus driving the demand for non-invasive monitoring solutions.Tell us your focus area and get a customized research report.
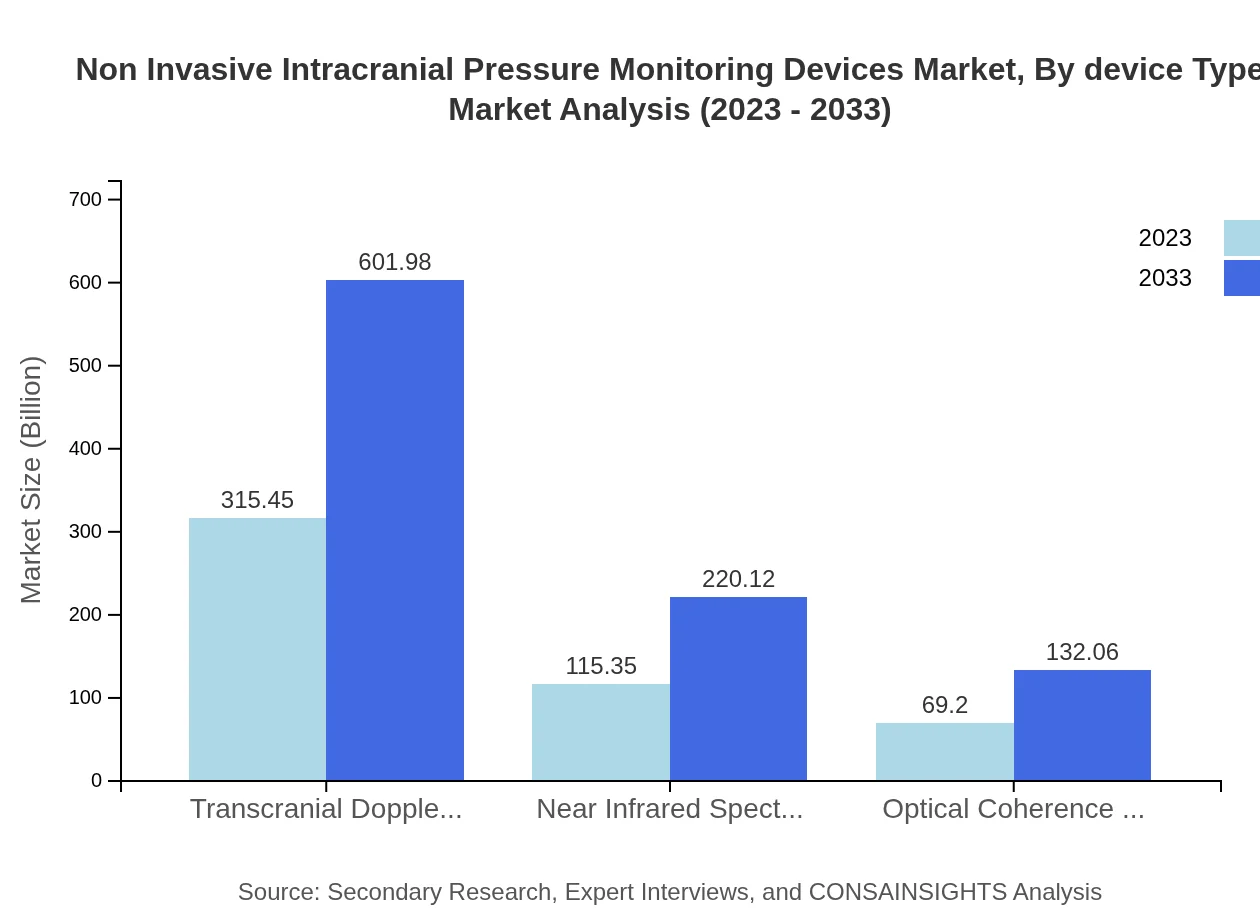
Non Invasive Intracranial Pressure Monitoring Devices Market Analysis By Device Type
The Non-Invasive Intracranial Pressure Monitoring Devices market by device type is classified mainly into Doppler Technology, NIRS Technology, and OCT Technology. Doppler devices currently dominate the market, with a substantial share and projected growth due to their widespread adoption in hospitals. NIRS and OCT devices follow, gaining traction due to technological advancements offering improved accuracy and real-time monitoring capabilities.
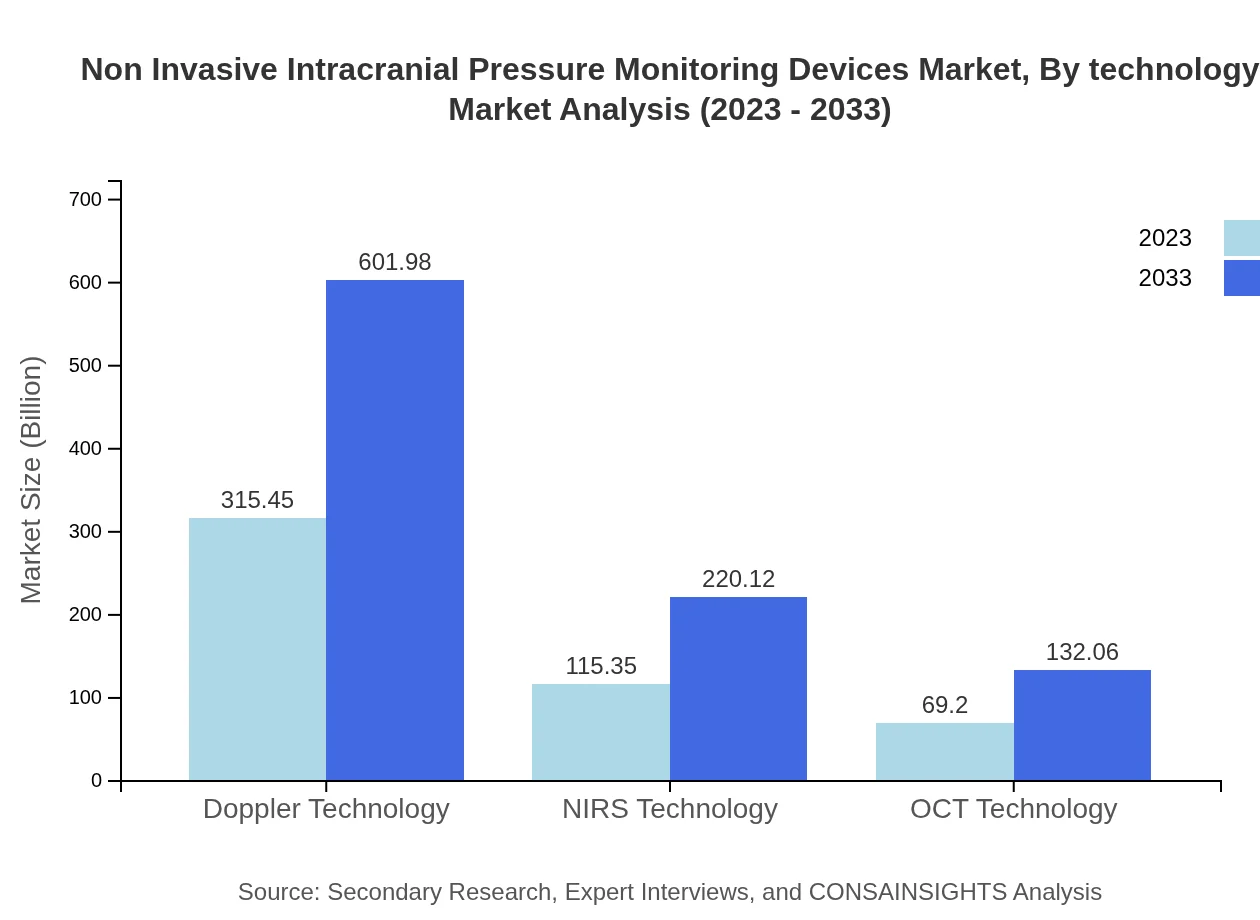
Non Invasive Intracranial Pressure Monitoring Devices Market Analysis By Technology
Market segmentation by technology highlights the effectiveness of various methodologies such as Transcranial Doppler, Near Infrared Spectroscopy, and Optical Coherence Tomography used for measuring ICP. These technologies differ in precision, ease of use, and applicability in different clinical scenarios. Transcranial Doppler remains the preferred choice among practitioners for its ease of use and non-invasive nature.
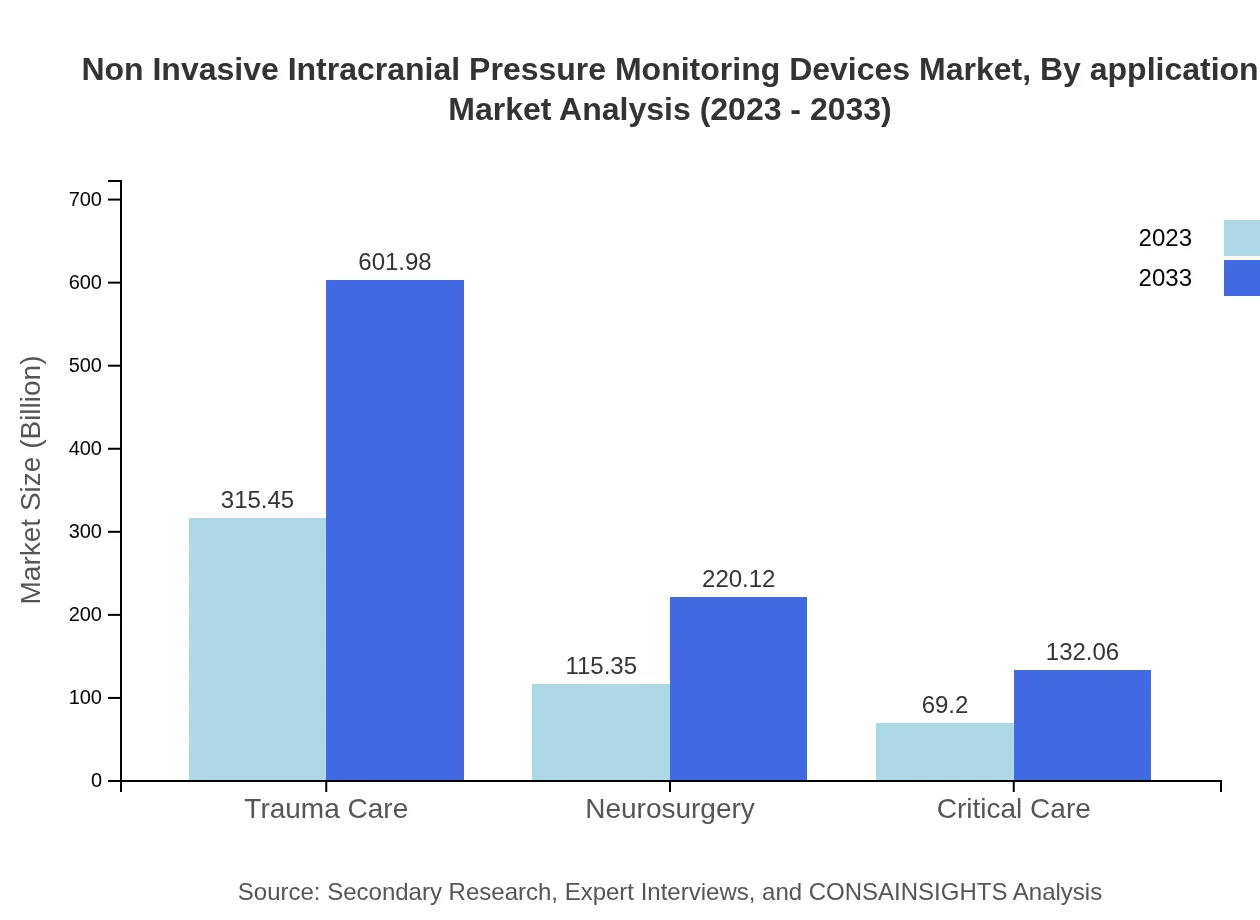
Non Invasive Intracranial Pressure Monitoring Devices Market Analysis By Application
The applications of Non-Invasive ICP Monitoring Devices span across trauma care, critical care, and neurosurgery. Trauma care leads in market share due to the high prevalence of TBIs and the urgent need for accurate monitoring. Critical care units are increasingly adopting these devices, emphasizing the importance of continuous ICP monitoring in high-risk patients.
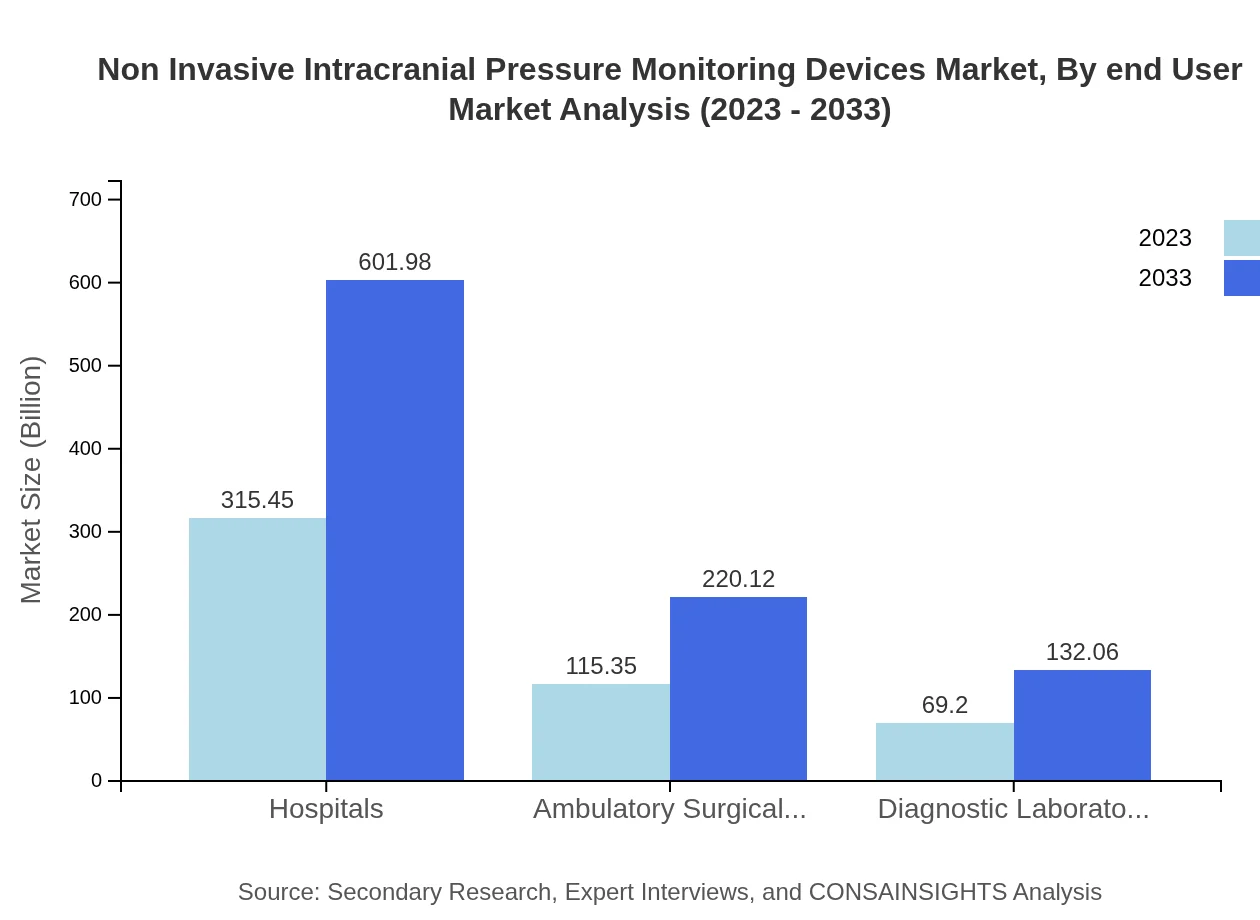
Non Invasive Intracranial Pressure Monitoring Devices Market Analysis By End User
Hospitals constitute the largest end-user segment for Non-Invasive ICP Monitoring Devices, expected to maintain dominance due to their extensive use in emergency and critical care settings, contributing to over 60% of the overall market. Ambulatory surgical centers are gaining prominence, reflecting a trend toward outpatient management of neurological conditions.
Non Invasive Intracranial Pressure Monitoring Devices Market Analysis By Region
Regional analysis reveals North America as the leading market, due to advanced healthcare infrastructure and high incidence rates of neurological diseases. Europe follows closely, spearheaded by Germany and the UK, while the Asia Pacific region showcases the fastest growth owing to investments in healthcare and technology advancement, particularly in China and India.
Non Invasive Intracranial Pressure Monitoring Devices Market Trends and Future Forecast
Tell us your focus area and get a customized research report.
Global Market Leaders and Top Companies in Non Invasive Intracranial Pressure Monitoring Devices Industry
Natus Medical Incorporated:
Natus Medical is a leader in the development of innovative healthcare solutions for brain and spine care, providing advanced ICP monitoring devices that cater to critical need areas in pediatric and adult populations.Medtronic :
Medtronic develops a range of non-invasive neurological devices aimed at improving patient outcomes, backing their products with robust clinical evidence and extensive market presence.Cerebrotech Medical Systems, Inc.:
Cerebrotech focuses on innovative technologies for brain health monitoring, with non-invasive ICP monitoring systems designed for ease of use in both clinical and emergency settings.Raumedic AG:
Raumedic specializes in high-quality medical devices, contributing advanced non-invasive solutions for monitoring intracranial pressure and providing solutions for neurocritical care.We're grateful to work with incredible clients.









FAQs
What is the market size of Non-Invasive Intracranial Pressure Monitoring Devices?
The non-invasive intracranial pressure monitoring devices market is valued at approximately $500 million in 2023, with an anticipated CAGR of 6.5% until 2033, signaling steady growth and increasing adoption of these technologies.
What are the key market players or companies in the Non-Invasive Intracranial Pressure Monitoring Devices industry?
Key players in this market include leading medical technology companies, innovative startups focusing on neuromonitoring, and firms specializing in diagnostic and therapeutic devices. These companies are instrumental in technological advancements and market expansion.
What are the primary factors driving the growth in the Non-Invasive Intracranial Pressure Monitoring Devices industry?
The growth is driven by rising incidences of neurological disorders, increased awareness about non-invasive measurements, advancements in technology, and the growing preference for cost-effective and safer monitoring solutions in healthcare settings.
Which region is the fastest Growing in the Non-Invasive Intracranial Pressure Monitoring Devices market?
Asia-Pacific is the fastest-growing region, projecting growth from $88.15 million in 2023 to $168.22 million by 2033. This growth is attributed to increasing healthcare expenditures and advancements in medical technology in emerging markets.
Does Consainsights provide customized market report data for the Non-Invasive Intracranial Pressure Monitoring Devices industry?
Yes, Consainsights offers customized market reports tailored to specific needs, allowing clients to obtain data relevant to their strategic objectives, including geographical insights and competitive analysis.
What deliverables can I expect from this Non-Invasive Intracranial Pressure Monitoring Devices market research project?
Expected deliverables include comprehensive market analysis reports, segmentation data, market shares of players, regional analysis, forecasts, and insights into industry trends that support informed business decisions.
What are the market trends of Non-Invasive Intracranial Pressure Monitoring Devices?
Current market trends include increasing collaboration between tech firms and healthcare providers, enhancements in device functionality, growing adoption of telemedicine, and a shift towards integrated health solutions in critical care management.