Non Invasive Prenatal Testing Market Report
Published Date: 31 January 2026 | Report Code: non-invasive-prenatal-testing
Non Invasive Prenatal Testing Market Size, Share, Industry Trends and Forecast to 2033
This report provides a comprehensive analysis of the Non Invasive Prenatal Testing (NIPT) market from 2023 to 2033, highlighting market dynamics, size, trends, and forecasts alongside regional and segment analysis.
| Metric | Value |
|---|---|
| Study Period | 2023 - 2033 |
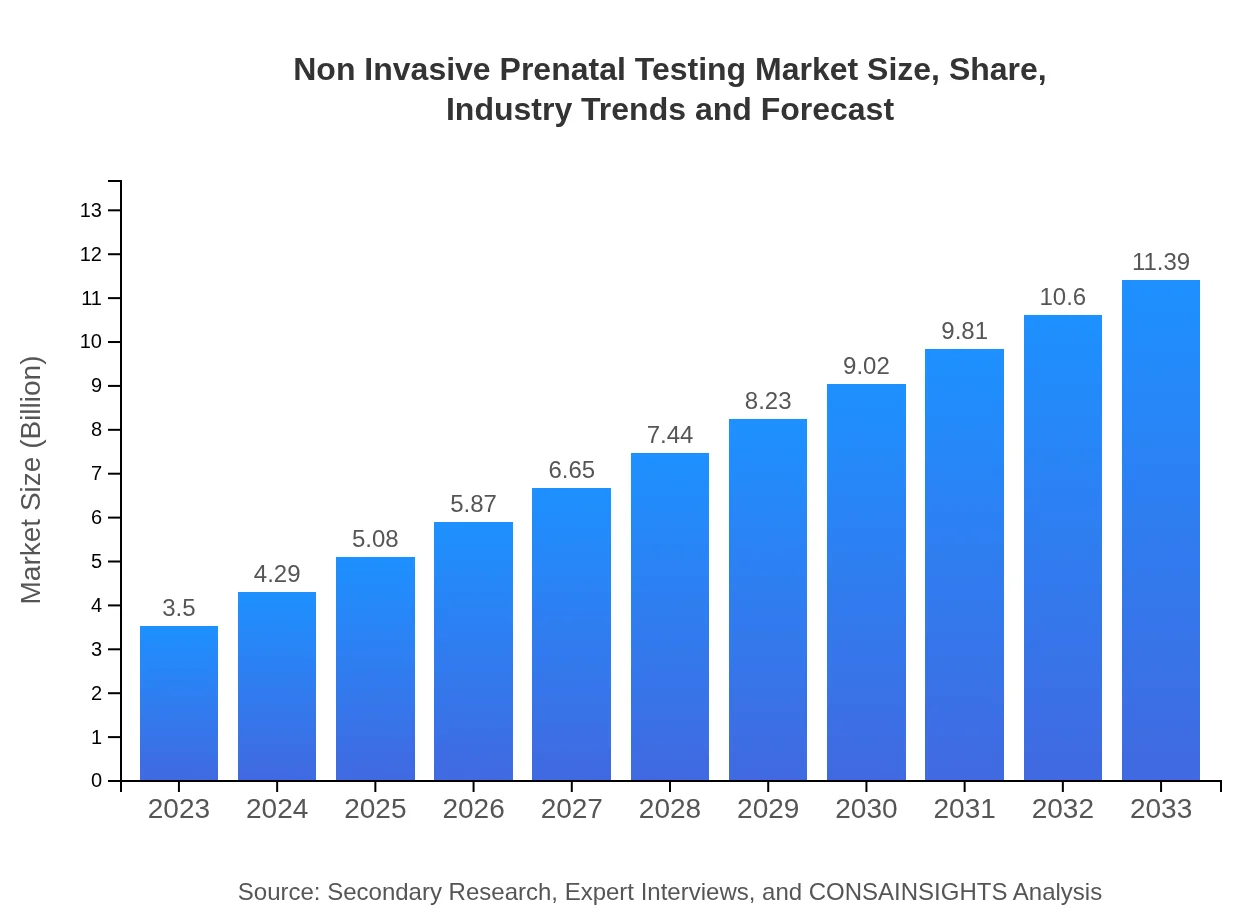
| 2023 Market Size | $3.50 Billion |
| CAGR (2023-2033) | 12% |
| 2033 Market Size | $11.39 Billion |
| Top Companies | Illumina, Inc., Roche Diagnostics, Natera, Inc., Sequenom, Inc. |
| Last Modified Date | 31 January 2026 |
Non Invasive Prenatal Testing Market Overview
Customize Non Invasive Prenatal Testing Market Report market research report
- ✔ Get in-depth analysis of Non Invasive Prenatal Testing market size, growth, and forecasts.
- ✔ Understand Non Invasive Prenatal Testing's regional dynamics and industry-specific trends.
- ✔ Identify potential applications, end-user demand, and growth segments in Non Invasive Prenatal Testing
What is the Market Size & CAGR of Non Invasive Prenatal Testing market in 2033?
Non Invasive Prenatal Testing Industry Analysis
Non Invasive Prenatal Testing Market Segmentation and Scope
Tell us your focus area and get a customized research report.
Non Invasive Prenatal Testing Market Analysis Report by Region
Europe Non Invasive Prenatal Testing Market Report:
In Europe, the NIPT market is set to grow from $0.91 billion in 2023 to $2.95 billion by 2033. Factors such as robust healthcare policies, increased adoption of NIPT in prenatal care, and a rising prevalence of genetic disorders contribute to this growth.Asia Pacific Non Invasive Prenatal Testing Market Report:
The Asia Pacific region is expected to exhibit substantial growth, with a market size projected to reach $2.25 billion by 2033, up from $0.69 billion in 2023. This growth is driven by increasing healthcare expenditure, rising awareness of prenatal testing, and improvements in healthcare infrastructure.North America Non Invasive Prenatal Testing Market Report:
North America holds a significant share of the NIPT market, projected to expand from $1.26 billion in 2023 to $4.08 billion by 2033. The high adoption rate of advanced diagnostic technologies and a well-established healthcare system are key growth drivers.South America Non Invasive Prenatal Testing Market Report:
The South America NIPT market is anticipated to grow from $0.16 billion in 2023 to $0.53 billion by 2033, fostered by improving access to healthcare and increasing emphasis on maternal health initiatives.Middle East & Africa Non Invasive Prenatal Testing Market Report:
The Middle East and Africa NIPT market is projected to grow from $0.48 billion in 2023 to $1.57 billion by 2033, propelled by initiatives to enhance maternal health services and growing numbers of healthcare facilities.Tell us your focus area and get a customized research report.
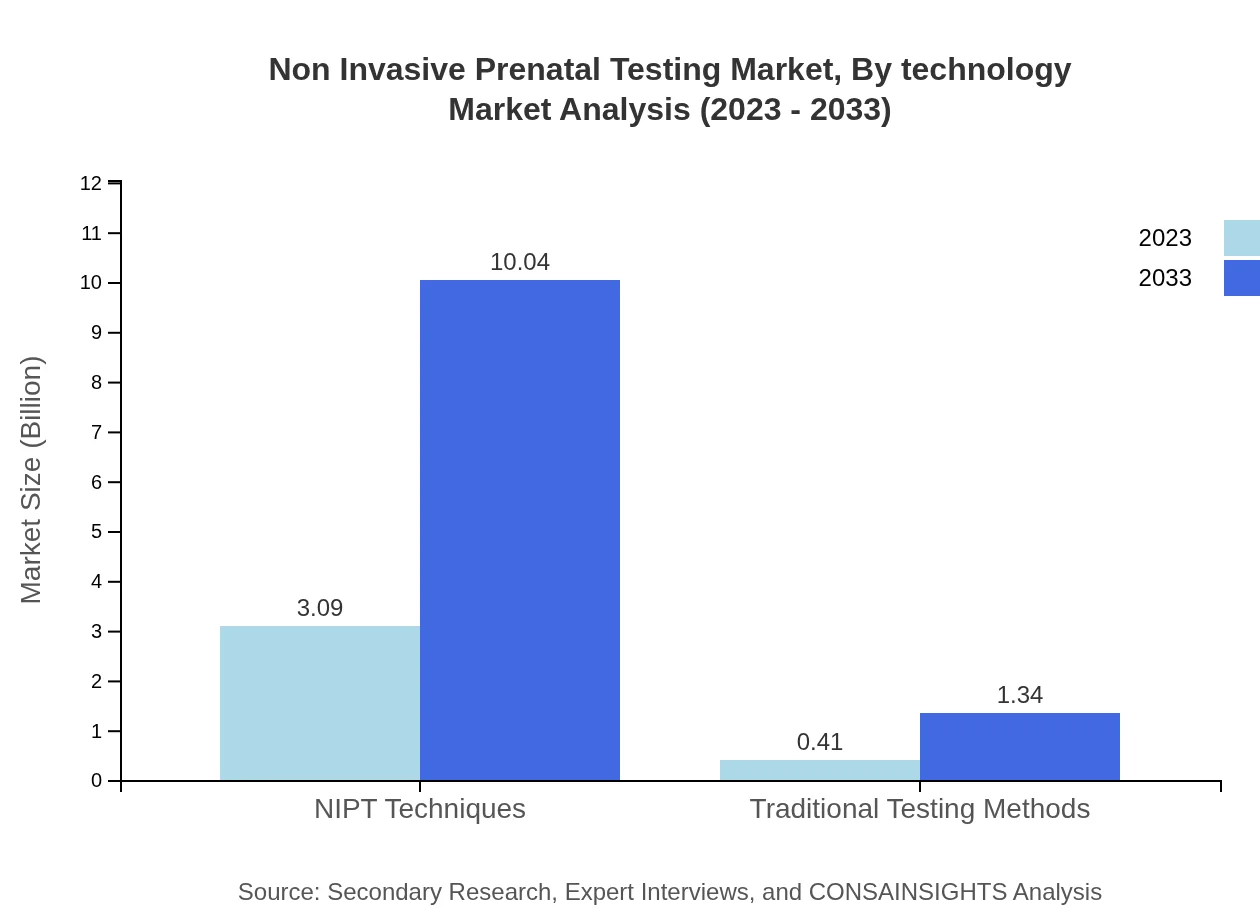
Non Invasive Prenatal Testing Market Analysis By Technology
The NIPT market, when analyzed by technology, shows that testing kits dominate the landscape with a projected market size reaching $10.04 billion by 2033. Their popularity is attributed to their convenience and accuracy in prenatal screening, while software solutions are anticipated to contribute significantly as complementary tools enhancing diagnostic capabilities.
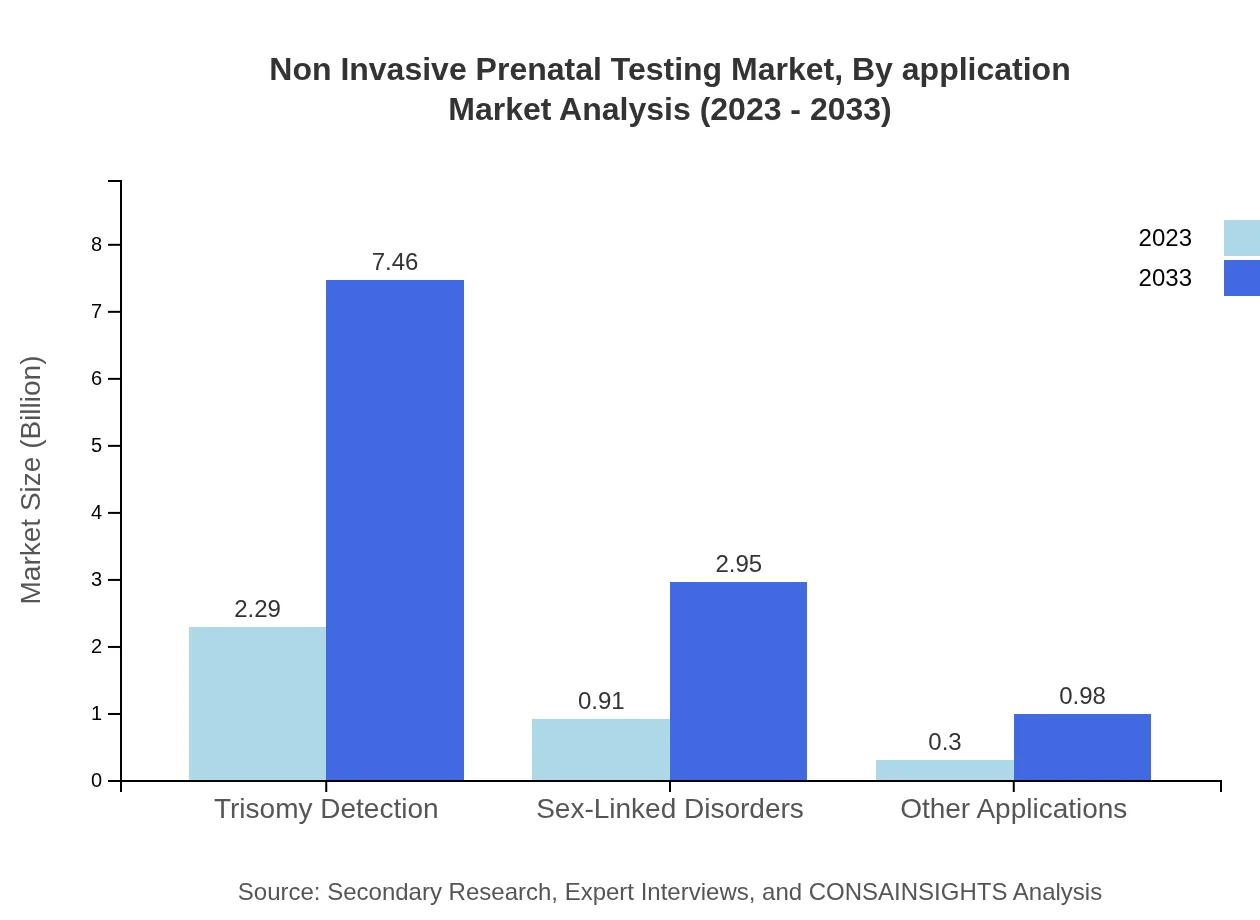
Non Invasive Prenatal Testing Market Analysis By Application
Market segmentation based on application reveals a strong preference for hospitals which are expected to command a market size of $7.46 billion by 2033, growing from $2.29 billion in 2023. Hospitals are key players due to the volume of testing conducted and their focus on providing comprehensive maternal-fetal health services.
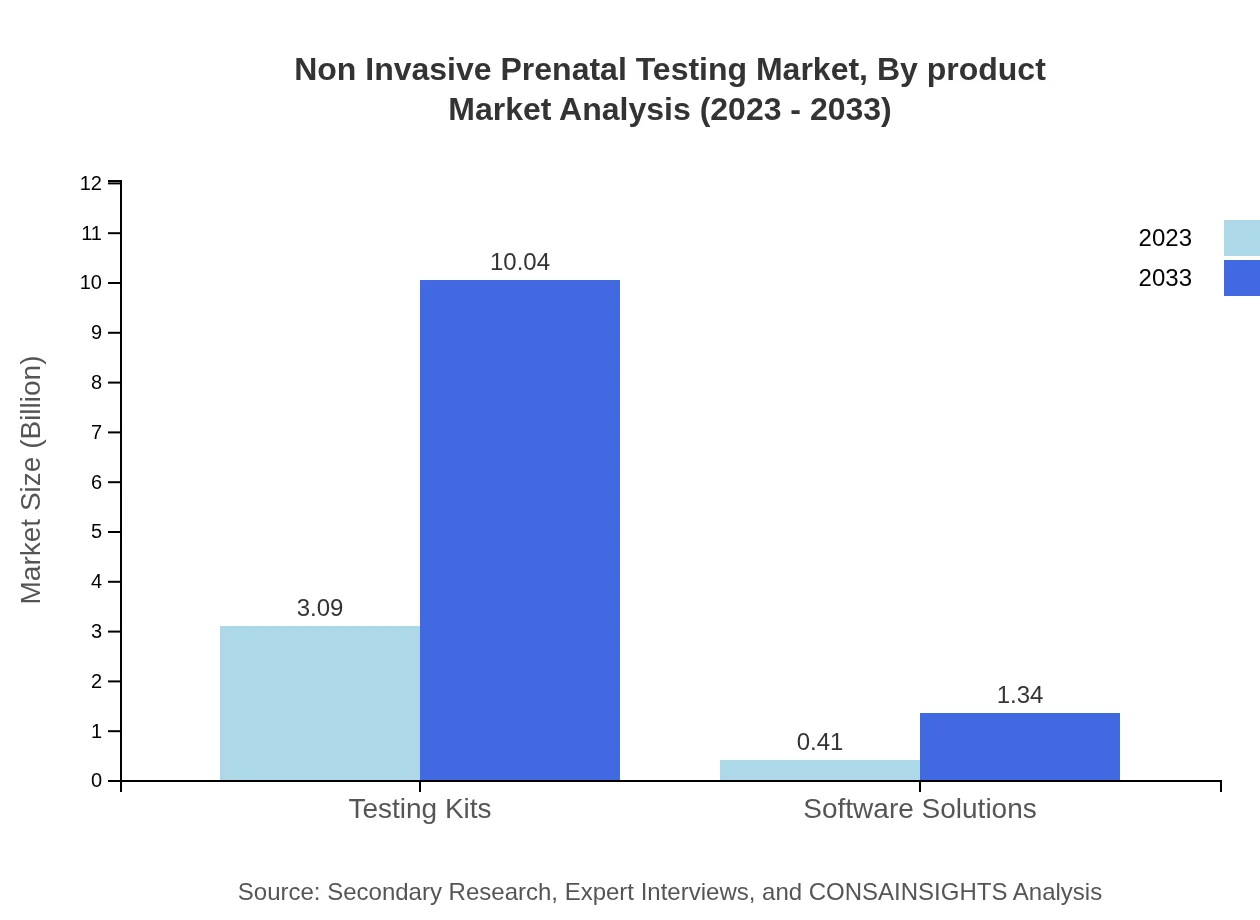
Non Invasive Prenatal Testing Market Analysis By Product
In product segmentation, testing kits occupy the majority share and translate to a market size of $10.04 billion by 2033, reflecting sustained demand driven by innovations in prenatal testing technologies.
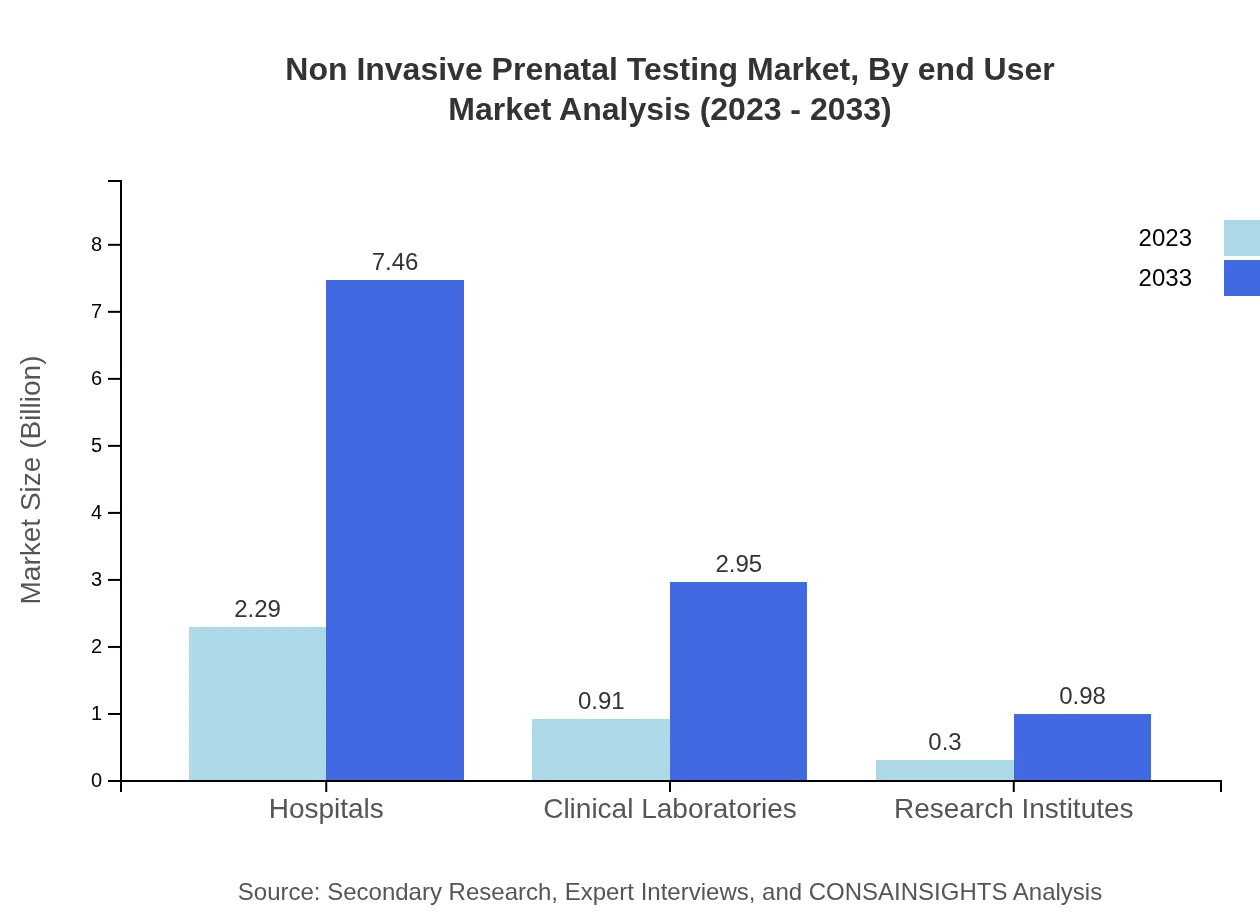
Non Invasive Prenatal Testing Market Analysis By End User
When analyzing by end-users, hospitals lead in market share, expected to reach $7.46 billion by 2033. Clinical laboratories are also significant contributors, projected to grow to a size of $2.95 billion by the same year, as their role in processing and validating NIPT expands.
Non Invasive Prenatal Testing Market Analysis By Region Application
Regional segmentation emphasizes distinct market dynamics, with North America leading not only in size but also in application range. Europe and the Asia Pacific are emerging as strong contenders due to increased awareness and investments in maternal health services.
Non Invasive Prenatal Testing Market Trends and Future Forecast
Tell us your focus area and get a customized research report.
Global Market Leaders and Top Companies in Non Invasive Prenatal Testing Industry
Illumina, Inc.:
A leader in genomics and DNA sequencing technology, Illumina produces cutting-edge NIPT solutions that enhance prenatal screening efficiency and accuracy.Roche Diagnostics:
A prominent player in molecular diagnostics, Roche offers comprehensive NIPT tests providing critical insights into maternal and fetal health.Natera, Inc.:
Natera specializes in genetic testing and offers advanced NIPT solutions that cater to various prenatal screening needs.Sequenom, Inc.:
Sequenom is known for its innovative NIPT technologies that help in the early detection of genetic abnormalities during pregnancy.We're grateful to work with incredible clients.









FAQs
What is the market size of non Invasive prenatal testing?
The non-invasive prenatal testing market is projected to grow from $3.5 billion in 2023 to approximately $13 billion by 2033, with a compound annual growth rate (CAGR) of 12% over the forecast period.
What are the key market players or companies in this non Invasive prenatal testing industry?
Key players in the non-invasive prenatal testing industry include Illumina, Roche, Natera, Myriad Genetics, and Ariosa Diagnostics, among others, known for innovation and significant contributions to market development.
What are the primary factors driving the growth in the non Invasive prenatal testing industry?
Growth in the non-invasive prenatal testing industry is driven by rising awareness of prenatal health, increasing adoption of advanced technologies, and growing demand for safe testing options that reduce risks associated with invasive procedures.
Which region is the fastest Growing in the non Invasive prenatal testing?
Asia Pacific is observed as the fastest-growing region for non-invasive prenatal testing, expected to expand from $0.69 billion in 2023 to $2.25 billion by 2033, driven by rising healthcare investments and growing maternal health awareness.
Does ConsaInsights provide customized market report data for the non Invasive prenatal testing industry?
Yes, ConsaInsights offers customized market report data tailored to the specific needs and requirements of stakeholders in the non-invasive prenatal testing industry, ensuring relevant insights and strategic guidance.
What deliverables can I expect from this non Invasive prenatal testing market research project?
Deliverables from the non-invasive prenatal testing market research project typically include comprehensive market analysis reports, segment insights, regional trends, competitive landscape evaluations, and strategic recommendations.
What are the market trends of non Invasive prenatal testing?
Current market trends in non-invasive prenatal testing include a shift towards personalized medicine, increased implementation of technology in prenatal diagnostics, and a growing focus on early detection of genetic disorders.