Non Vascular Stents Market Report
Published Date: 31 January 2026 | Report Code: non-vascular-stents
Non Vascular Stents Market Size, Share, Industry Trends and Forecast to 2033
This report provides a comprehensive analysis of the Non Vascular Stents market, covering market size, growth forecasts, segmentations, and industry trends from 2023 to 2033. Insights into regional dynamics and key players are also included to guide stakeholders in making informed decisions.
| Metric | Value |
|---|---|
| Study Period | 2023 - 2033 |
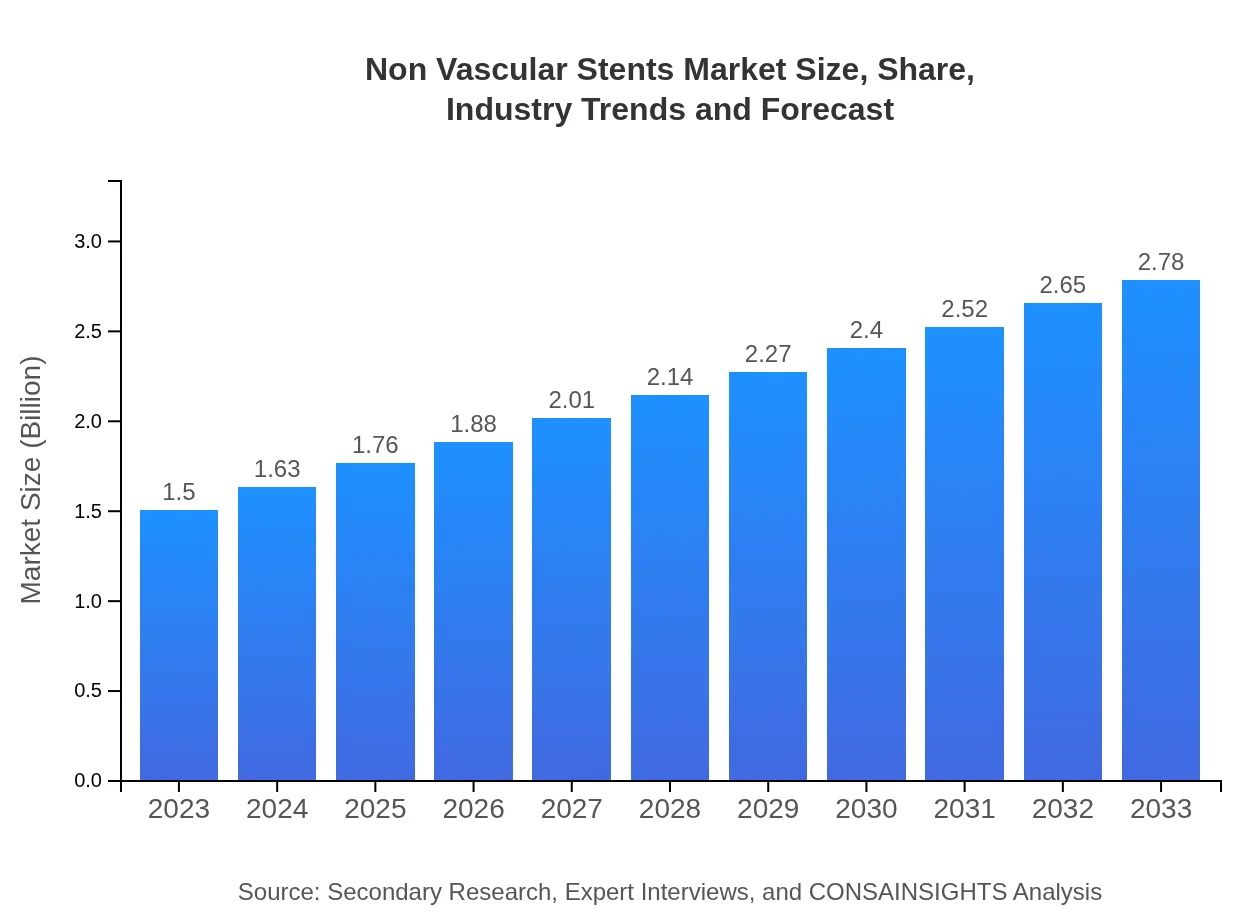
| 2023 Market Size | $1.50 Billion |
| CAGR (2023-2033) | 6.2% |
| 2033 Market Size | $2.78 Billion |
| Top Companies | Boston Scientific, Medtronic , Abbott Laboratories, C. R. Bard |
| Last Modified Date | 31 January 2026 |
Non Vascular Stents Market Overview
Customize Non Vascular Stents Market Report market research report
- ✔ Get in-depth analysis of Non Vascular Stents market size, growth, and forecasts.
- ✔ Understand Non Vascular Stents's regional dynamics and industry-specific trends.
- ✔ Identify potential applications, end-user demand, and growth segments in Non Vascular Stents
What is the Market Size & CAGR of Non Vascular Stents market in 2023?
Non Vascular Stents Industry Analysis
Non Vascular Stents Market Segmentation and Scope
Tell us your focus area and get a customized research report.
Non Vascular Stents Market Analysis Report by Region
Europe Non Vascular Stents Market Report:
The European market for Non Vascular Stents is expected to grow from $0.47 billion in 2023 to $0.86 billion by 2033. This growth can be attributed to well-established healthcare infrastructure and increasing preferences for minimally invasive surgical procedures.Asia Pacific Non Vascular Stents Market Report:
In the Asia Pacific region, the Non Vascular Stents market is valued at approximately $0.27 billion in 2023, projected to grow to $0.49 billion by 2033. The growth is driven by increasing healthcare expenditures and rising awareness of stenting technologies, particularly in countries like India and China.North America Non Vascular Stents Market Report:
In North America, the market is anticipated to grow from $0.57 billion in 2023 to $1.06 billion in 2033. The region benefits from advanced healthcare systems, high adoption rates for new technologies, and strong research and development initiatives.South America Non Vascular Stents Market Report:
The South American market for Non Vascular Stents shows a stable landscape with sales remaining at $0.01 billion throughout the forecast period. Limited growth is expected due to economic uncertainties and lower healthcare investments.Middle East & Africa Non Vascular Stents Market Report:
The Middle East and Africa region is expected to see growth from $0.19 billion in 2023 to $0.35 billion by 2033. The increase is fueled by improving healthcare services and rising incidences of relevant disease conditions warranting stenting.Tell us your focus area and get a customized research report.
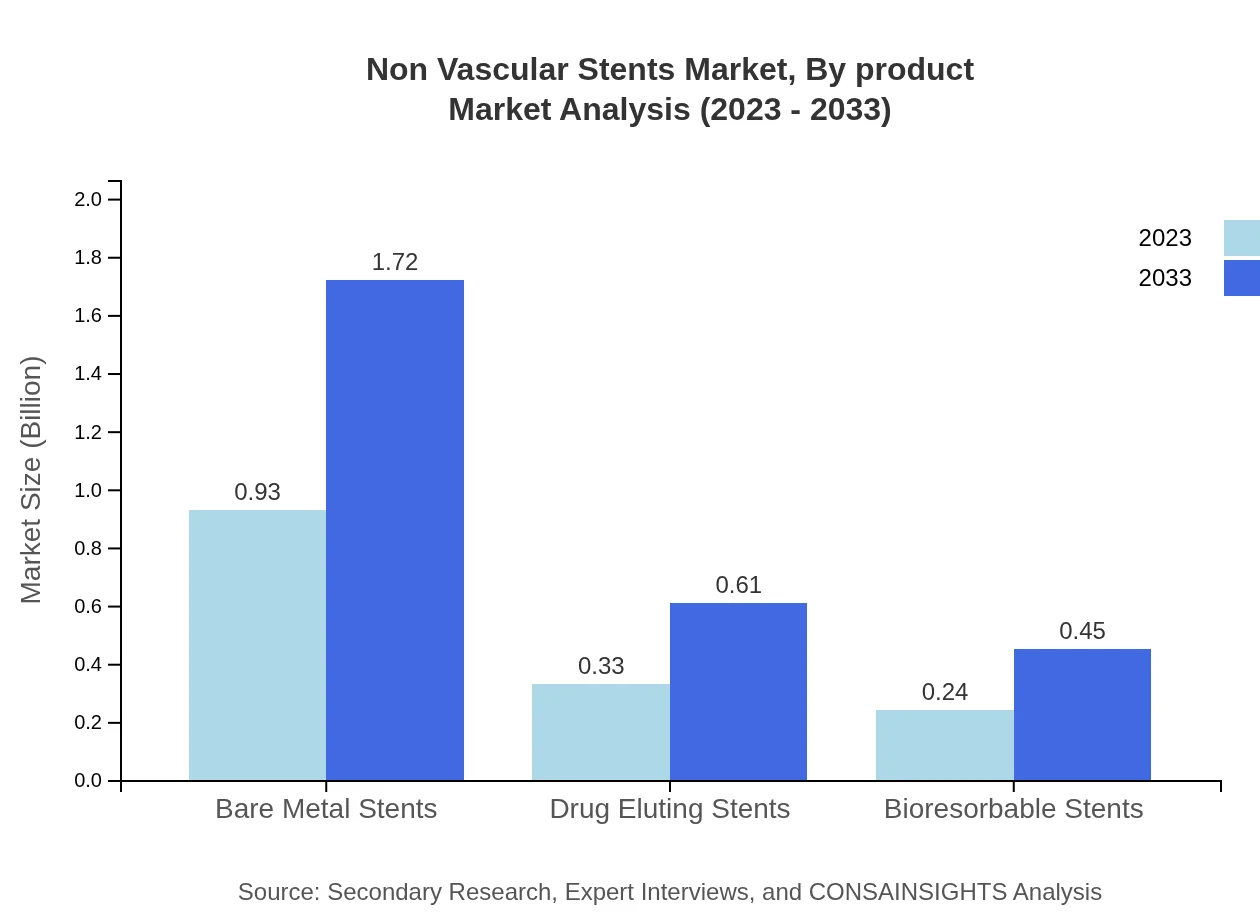
Non Vascular Stents Market Analysis By Product
The Non-Vascular Stents Market is dominated by temporary stents which accounted for $1.31 billion in 2023 and are expected to rise to $2.43 billion by 2033, capturing 87.37% market share. Permanent stents, on the other hand, represent a smaller segment valued at $0.19 billion in 2023, growing to $0.35 billion by 2033 and maintaining a 12.63% share.
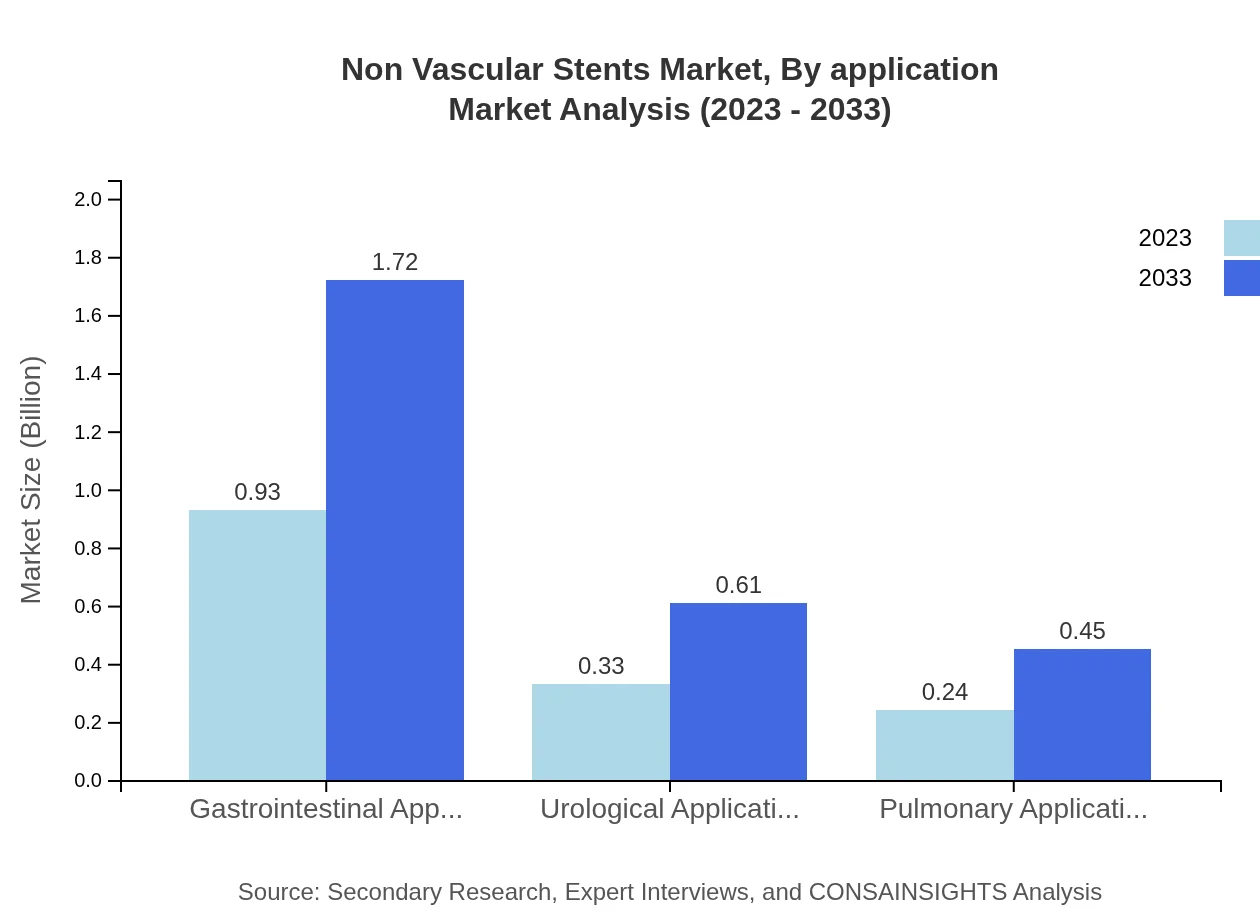
Non Vascular Stents Market Analysis By Application
The key applications for Non-Vascular Stents include gastrointestinal, urological, and pulmonary applications. Gastrointestinal applications account for the largest market size of $0.93 billion in 2023, expected to grow to $1.72 billion by 2033. Urological applications currently stand at $0.33 billion, rising to $0.61 billion in the same period, while pulmonary applications will grow from $0.24 billion to $0.45 billion by 2033.
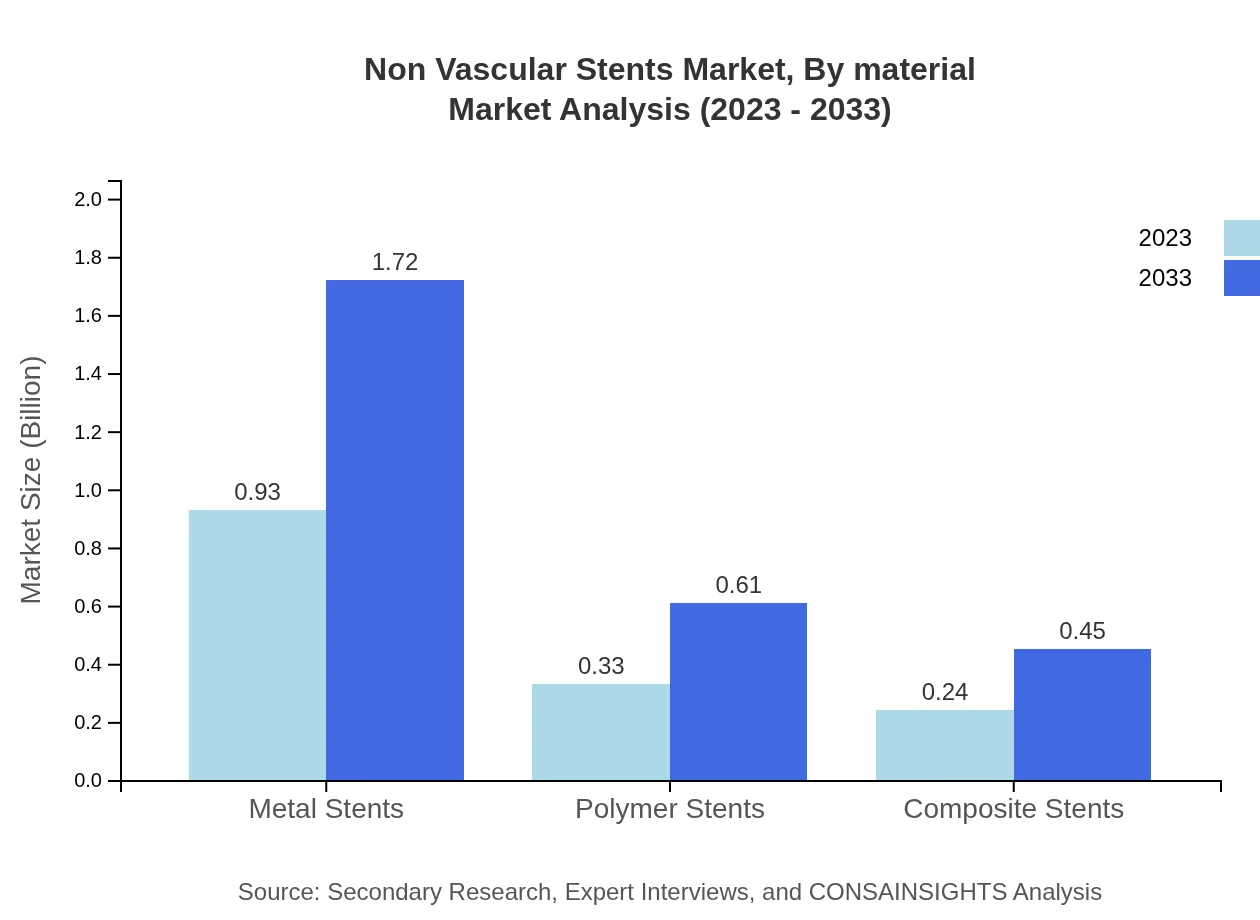
Non Vascular Stents Market Analysis By Material
Metal stents are the leading material choice, dominating the market with $0.93 billion in 2023 and forecasted to reach $1.72 billion by 2033 with a 61.88% share. Followed by polymer stents at $0.33 billion in 2023, expected to grow to $0.61 billion, and bioresorbable stents which are estimated to increase from $0.24 billion to $0.45 billion.
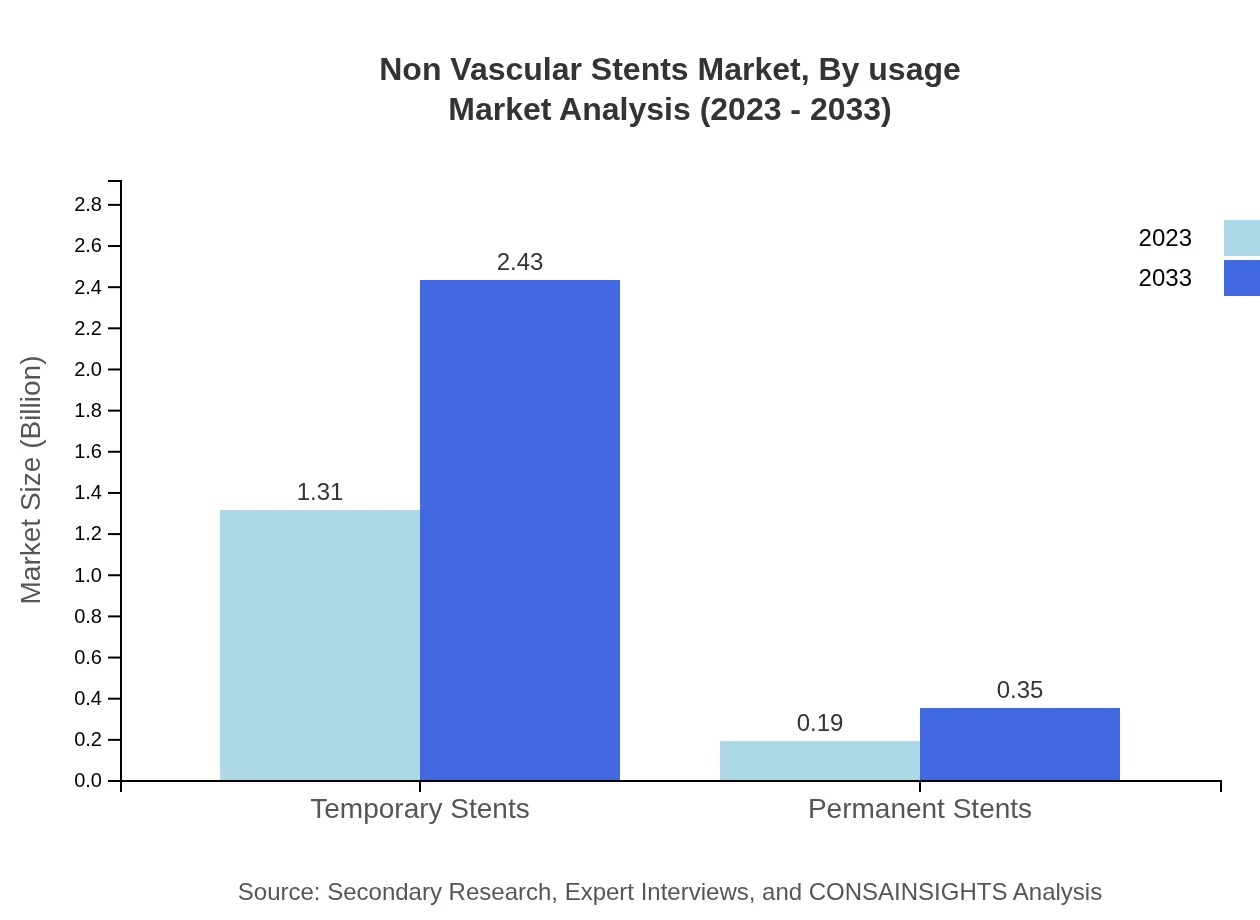
Non Vascular Stents Market Analysis By Usage
The usage of Non-Vascular Stents is primarily bifurcated into temporary and permanent categories. Temporary stents are favored due to their effectiveness in acute conditions with a current market size of $1.31 billion projected to increase to $2.43 billion, while permanent stents are valued at $0.19 billion, growing to $0.35 billion.
Non Vascular Stents Market Trends and Future Forecast
Tell us your focus area and get a customized research report.
Global Market Leaders and Top Companies in Non Vascular Stents Industry
Boston Scientific:
Boston Scientific is a leading innovator in the medical device industry, providing a range of solutions including advanced stents for non-vascular applications that enhance patient outcomes.Medtronic :
Medtronic develops and manufactures stents, focusing on comprehensive treatment for a wide range of medical conditions with a strong emphasis on technology and patient safety.Abbott Laboratories:
Abbott Laboratories specializes in innovative medical devices and technologies, including numerous solutions for non-vascular stenting applications, ensuring quality and efficiency.C. R. Bard:
C. R. Bard is known for its medical products and technologies, leading in the non-vascular stent market with a focus on both advanced design and patient care.We're grateful to work with incredible clients.









FAQs
What is the market size of non Vascular stents?
The non-vascular stents market is valued at approximately $1.5 billion in 2023, with a projected CAGR of 6.2%. By 2033, the market is expected to attain significant growth, emphasizing the increasing demand for various stent technologies.
What are the key market players or companies in this non Vascular stents industry?
The non-vascular stents industry features prominent companies such as Medtronic, Boston Scientific, Cook Medical, and B. Braun Melsungen. These players are known for their innovative stent technologies and extensive portfolios in the market.
What are the primary factors driving the growth in the non Vascular stents industry?
Growth in the non-vascular stents industry is primarily driven by advancements in stent technology, increasing prevalence of gastrointestinal and urological diseases, and rising healthcare expenditure aimed at improving patient outcomes and procedures.
Which region is the fastest Growing in the non Vascular stents?
North America is the fastest-growing region in the non-vascular stents market, expected to grow from $0.57 billion in 2023 to $1.06 billion by 2033. This growth is fueled by increasing adoption of advanced medical technologies.
Does ConsaInsights provide customized market report data for the non Vascular stents industry?
Yes, ConsaInsights offers customized market report data for the non-vascular stents industry. Clients can request specific insights tailored to their needs, including competitive analysis and regional market breakdowns.
What deliverables can I expect from this non Vascular stents market research project?
Clients can expect comprehensive deliverables from this market research project, including detailed reports, segment analysis, regional insights, forecasts, and data visualizations, all designed to aid strategic decision-making.
What are the market trends of non Vascular stents?
Current trends in the non-vascular stents market include the rising demand for bioresorbable and drug-eluting stents, advancements in minimally invasive procedures, and increased focus on patient-centric designs and biocompatible materials.