Ntrk Fusion Gene Positive Advanced Solid Tumor Market Report
Published Date: 31 January 2026 | Report Code: ntrk-fusion-gene-positive-advanced-solid-tumor
Ntrk Fusion Gene Positive Advanced Solid Tumor Market Size, Share, Industry Trends and Forecast to 2033
This report provides an in-depth analysis of the Ntrk Fusion Gene positive advanced solid tumor market from 2023 to 2033, covering market size, growth forecast, regional insights, and key player contributions.
| Metric | Value |
|---|---|
| Study Period | 2023 - 2033 |
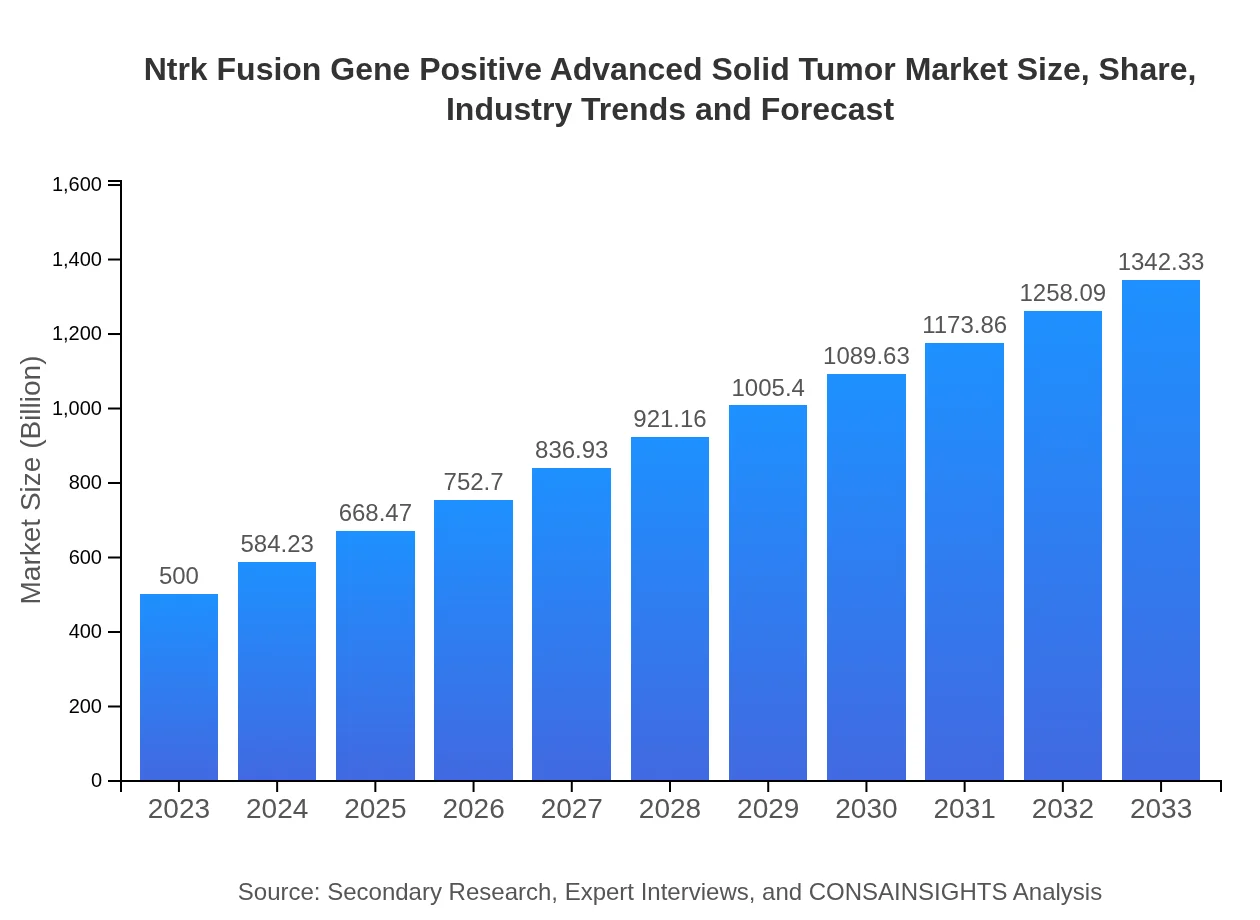
| 2023 Market Size | $500.00 Million |
| CAGR (2023-2033) | 10% |
| 2033 Market Size | $1342.33 Million |
| Top Companies | Bayer AG, Roche Holding AG, Blueprint Medicines, Loxo Oncology |
| Last Modified Date | 31 January 2026 |
Ntrk Fusion Gene Positive Advanced Solid Tumor Market Overview
Customize Ntrk Fusion Gene Positive Advanced Solid Tumor Market Report market research report
- ✔ Get in-depth analysis of Ntrk Fusion Gene Positive Advanced Solid Tumor market size, growth, and forecasts.
- ✔ Understand Ntrk Fusion Gene Positive Advanced Solid Tumor's regional dynamics and industry-specific trends.
- ✔ Identify potential applications, end-user demand, and growth segments in Ntrk Fusion Gene Positive Advanced Solid Tumor
What is the Market Size & CAGR of Ntrk Fusion Gene Positive Advanced Solid Tumor market in 2023?
Ntrk Fusion Gene Positive Advanced Solid Tumor Industry Analysis
Ntrk Fusion Gene Positive Advanced Solid Tumor Market Segmentation and Scope
Tell us your focus area and get a customized research report.
Ntrk Fusion Gene Positive Advanced Solid Tumor Market Analysis Report by Region
Europe Ntrk Fusion Gene Positive Advanced Solid Tumor Market Report:
Europe is forecasted to expand from $142.15 million in 2023 to $381.62 million in 2033. Increasing numbers of clinical trials and the region's emphasis on personalized medicine are enhancing market prospects. Collaboration between public and private sectors is further improving access to advanced Ntrk-targeted therapies.Asia Pacific Ntrk Fusion Gene Positive Advanced Solid Tumor Market Report:
The Asia Pacific region is projected to see substantial growth from $98.45 million in 2023 to $264.30 million in 2033. Factors include rising investment in healthcare infrastructure and increasing awareness of genetic testing among clinicians and patients. Innovative clinical trials targeting Ntrk fusions are emerging in this region, driving advancements in treatment options.North America Ntrk Fusion Gene Positive Advanced Solid Tumor Market Report:
North America maintains the largest market share, projected to increase from $184.05 million in 2023 to $494.11 million by 2033. The region benefits from a robust pharmaceutical landscape, strong investment in R&D, and high adoption rates of novel therapies. The US has several approved Ntrk inhibitors which form a significant portion of market revenue.South America Ntrk Fusion Gene Positive Advanced Solid Tumor Market Report:
The South American market is expected to grow from $39.45 million in 2023 to $105.91 million by 2033. Government initiatives aimed at improving cancer care and greater access to advanced therapies are contributing to this growth. Partnerships between local and global biotech firms are fostering research in oncology, specifically targeting solid tumors.Middle East & Africa Ntrk Fusion Gene Positive Advanced Solid Tumor Market Report:
The Middle East and Africa market is projected to grow from $35.90 million in 2023 to $96.38 million by 2033, driven by improvements in healthcare infrastructure and rising healthcare expenditure. Public health initiatives focused on cancer screening and genetic research aim to boost early detection and targeted treatment mechanisms.Tell us your focus area and get a customized research report.
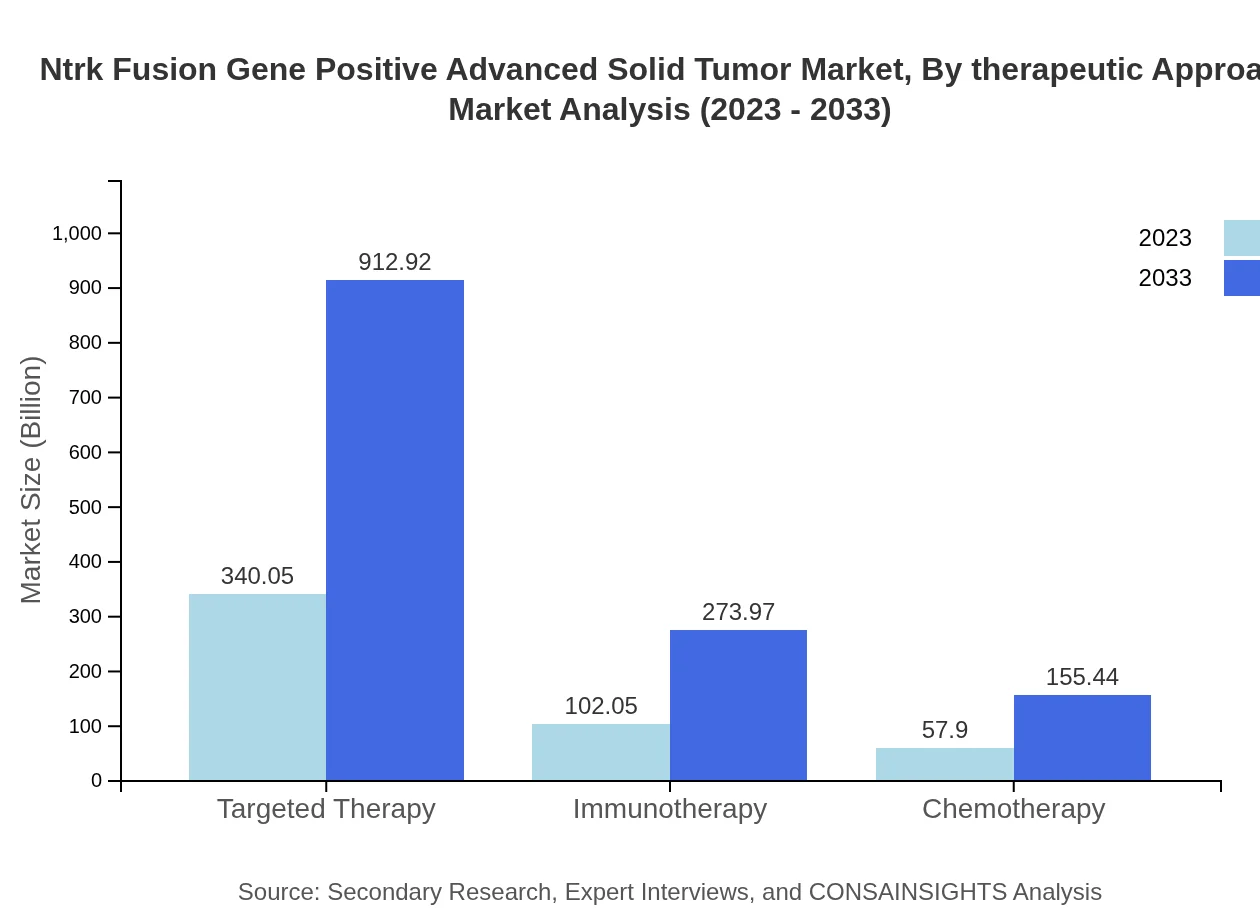
Ntrk Fusion Gene Positive Advanced Solid Tumor Market Analysis By Therapeutic Approach
The therapeutic approach segment is pivotal, with targeted therapy representing the largest share at $340.05 million in 2023 and projected to reach $912.92 million by 2033, maintaining a share of 68.01%. Immunotherapy and chemotherapy segments also show growth, with immunotherapy forecasted to rise from $102.05 million to $273.97 million over the same period.
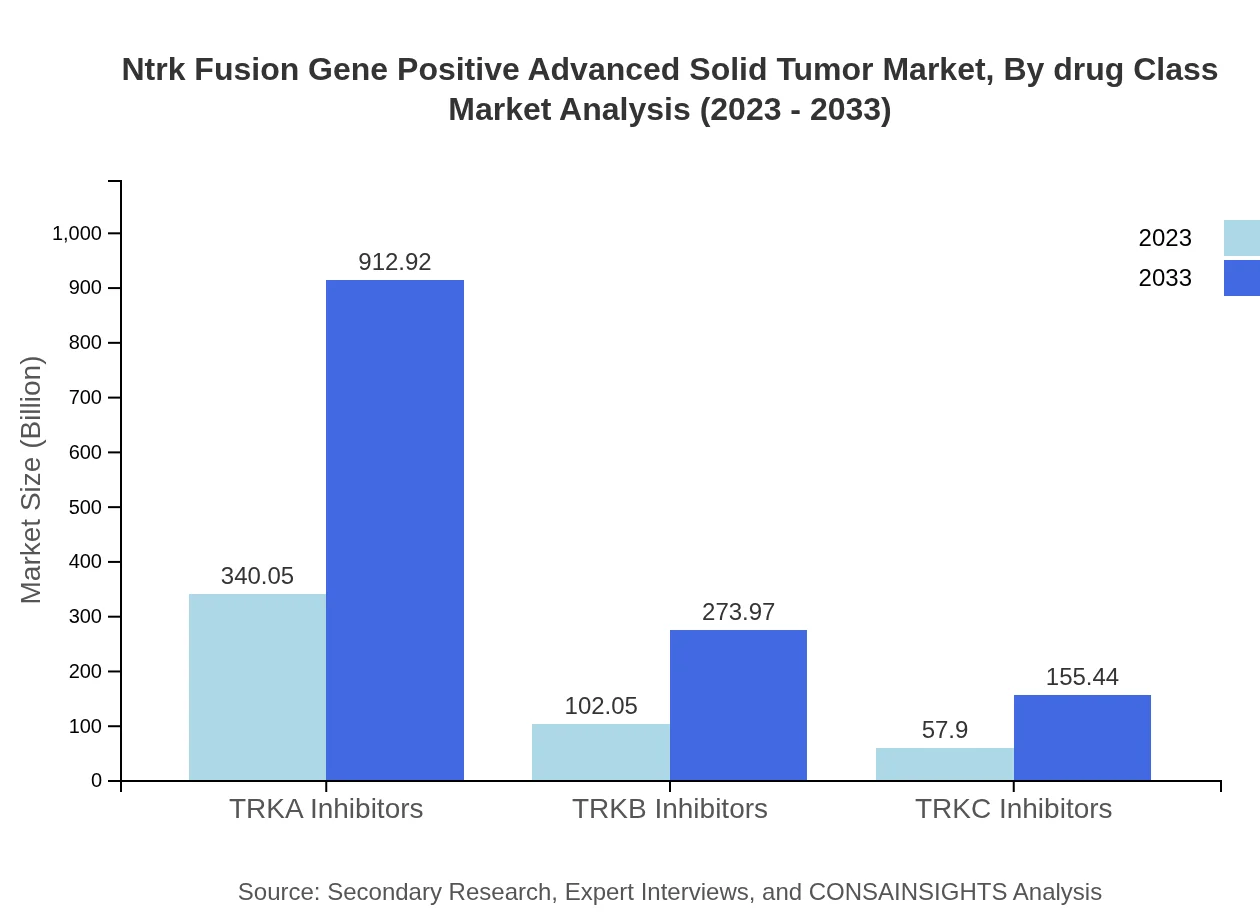
Ntrk Fusion Gene Positive Advanced Solid Tumor Market Analysis By Drug Class
The drug class segment showcases the prominence of TRKA inhibitors, which are expected to grow substantially from $340.05 million in 2023 to $912.92 million in 2033. TRKB inhibitors and TRKC inhibitors, although smaller in size, also exhibit strong growth rates, driven by clinical efficacy and research developments in targeted oncology.
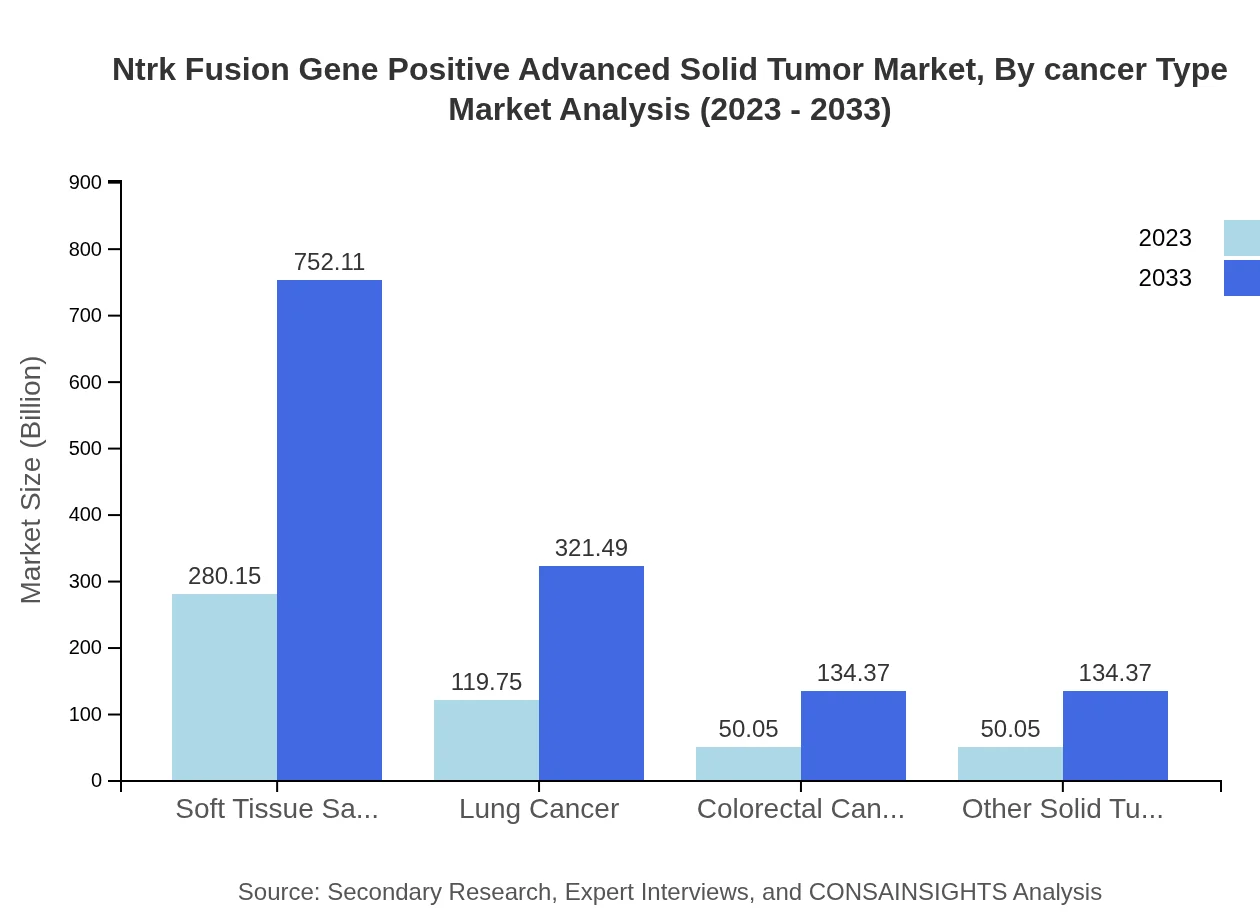
Ntrk Fusion Gene Positive Advanced Solid Tumor Market Analysis By Cancer Type
The market by cancer type is characterized by soft tissue sarcoma, which is projected to grow from $280.15 million in 2023 to $752.11 million by 2033. Lung cancer also represents a significant portion of the market, with growth driven by rising incidences of Ntrk fusions among lung cancer patients.
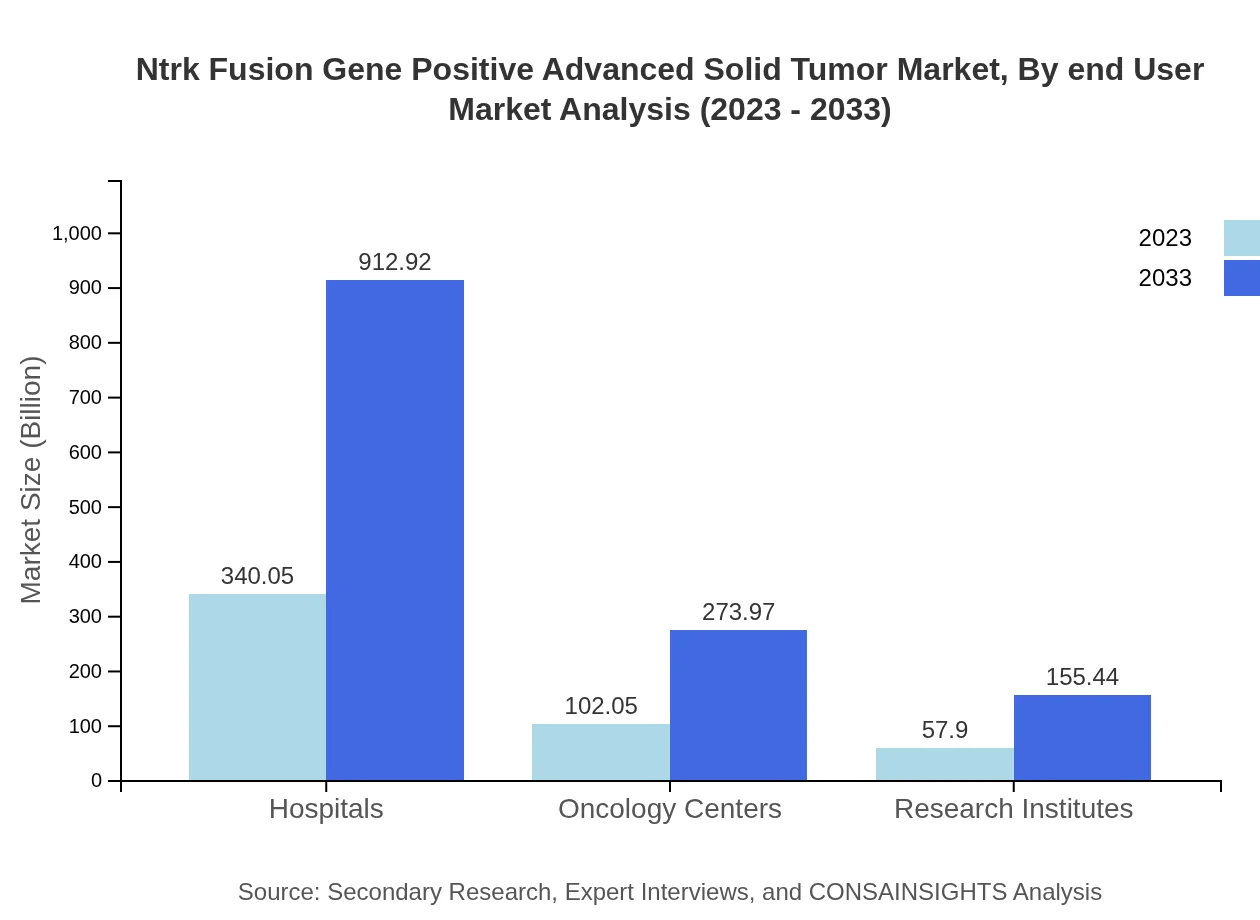
Ntrk Fusion Gene Positive Advanced Solid Tumor Market Analysis By End User
End-user segmentation indicates hospitals being the predominant market players, expanding from $340.05 million in 2023 to $912.92 million by 2033. Oncology centers and research institutions play vital roles in treatment delivery and ongoing clinical research, respectively.
Ntrk Fusion Gene Positive Advanced Solid Tumor Market Trends and Future Forecast
Tell us your focus area and get a customized research report.
Global Market Leaders and Top Companies in Ntrk Fusion Gene Positive Advanced Solid Tumor Industry
Bayer AG:
Bayer AG is known for its innovative approaches to targeted therapy, focusing on developing drugs for various cancer types including Ntrk fusions.Roche Holding AG:
Roche is a leader in the oncology space, offering breakthrough therapies that target Ntrk gene fusions, making significant impacts on treatment paradigms.Blueprint Medicines:
Blueprint Medicines specializes in targeted therapies for genomically defined cancers and has notable products for treating Ntrk fusion positive tumors.Loxo Oncology:
A subsidiary of Eli Lilly, Loxo Oncology focuses on developing innovative treatments for cancers with genetic drivers, including Ntrk fusion tumors.We're grateful to work with incredible clients.









FAQs
What is the market size of ntrk Fusion Gene Positive Advanced Solid Tumor?
The market size of the Ntrk Fusion Gene Positive Advanced Solid Tumor market is projected to reach approximately $500 million by 2033, growing at a CAGR of 10% from 2023. This growth reflects increasing investments in oncology research and development.
What are the key market players or companies in this ntrk Fusion Gene Positive Advanced Solid Tumor industry?
Key players in the Ntrk Fusion Gene Positive Advanced Solid Tumor market include major biotech and pharmaceutical companies specializing in targeted therapies and personalized medicine, significantly investing in developing innovative treatments for solid tumors.
What are the primary factors driving the growth in the ntrk Fusion Gene Positive Advanced Solid Tumor industry?
Growth is driven by rising cancer incidence, advancements in genetic testing and targeted therapies, increased awareness of personalized medicine, and favorable regulatory conditions enhancing research opportunities in this segment.
Which region is the fastest Growing in the ntrk Fusion Gene Positive Advanced Solid Tumor?
The fastest-growing region in the Ntrk Fusion Gene Positive Advanced Solid Tumor market is North America, expected to grow from $184.05 million in 2023 to $494.11 million by 2033, reflecting significant investments in biopharmaceutical research.
Does ConsaInsights provide customized market report data for the ntrk Fusion Gene Positive Advanced Solid Tumor industry?
Yes, Consainsights offers customized market report data tailored to specific needs in the Ntrk Fusion Gene Positive Advanced Solid Tumor industry, ensuring stakeholders receive insights suited to their strategic objectives.
What deliverables can I expect from this ntrk Fusion Gene Positive Advanced Solid Tumor market research project?
Deliverables from the Ntrk Fusion Gene Positive Advanced Solid Tumor market research project typically include detailed market analysis reports, growth forecasts, competitive landscape insights, and in-depth segmentation information across various parameters.
What are the market trends of ntrk Fusion Gene Positive Advanced Solid Tumor?
Market trends include a growing preference for targeted therapies, increasing clinical trials focused on Ntrk fusion genes, advancements in diagnostic technologies, and collaborations among biotech firms to develop innovative treatment solutions.