Nuclear Medicine Therapeutics Market Report
Published Date: 31 January 2026 | Report Code: nuclear-medicine-therapeutics
Nuclear Medicine Therapeutics Market Size, Share, Industry Trends and Forecast to 2033
This report provides a comprehensive analysis of the Nuclear Medicine Therapeutics market from 2023 to 2033, emphasizing market dynamics, size projections, trends, and competitive landscape insights, aimed to aid stakeholders in strategic decision-making.
| Metric | Value |
|---|---|
| Study Period | 2023 - 2033 |
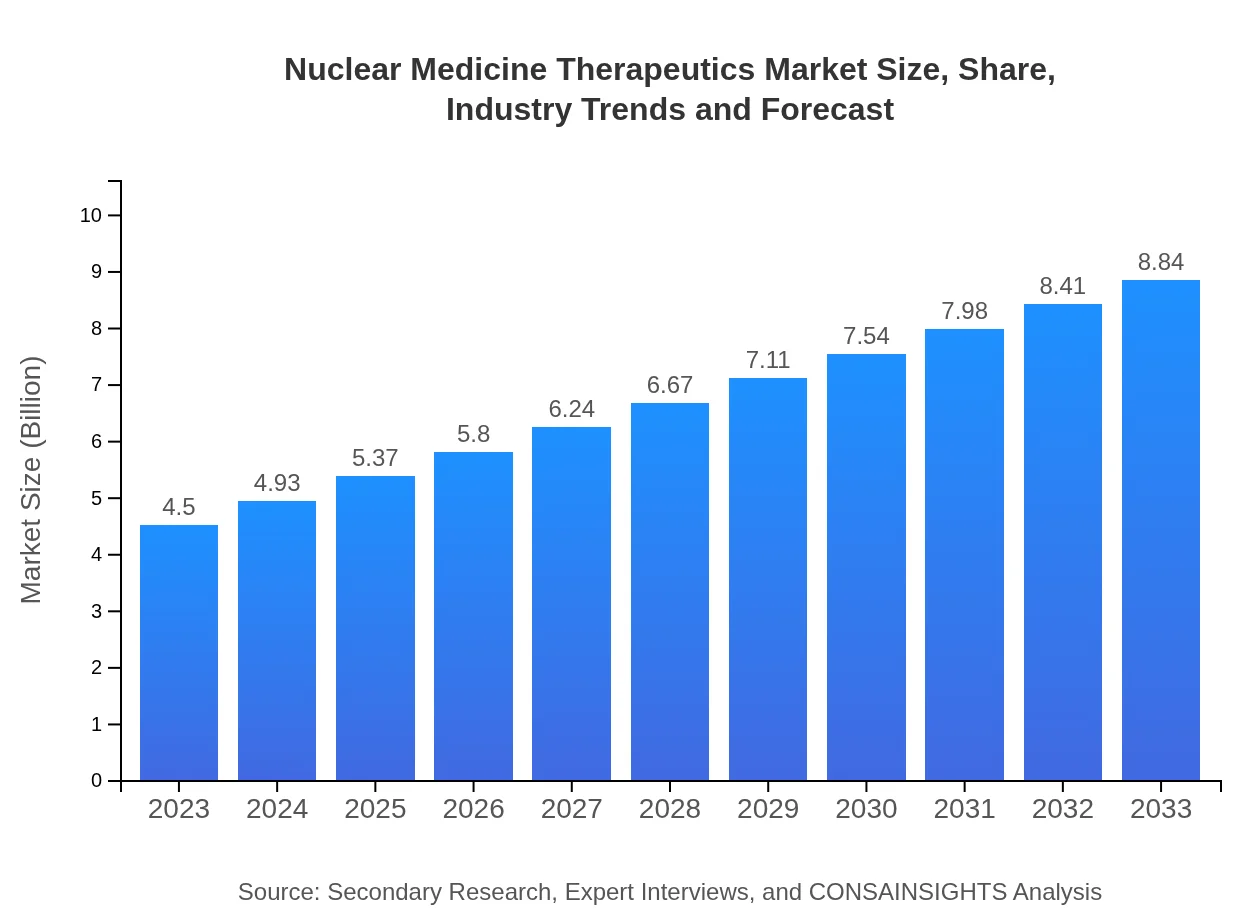
| 2023 Market Size | $4.50 Billion |
| CAGR (2023-2033) | 6.8% |
| 2033 Market Size | $8.84 Billion |
| Top Companies | General Electric Healthcare, Siemens Healthineers, Bayer AG, Novartis, Cardinal Health |
| Last Modified Date | 31 January 2026 |
Nuclear Medicine Therapeutics Market Overview
Customize Nuclear Medicine Therapeutics Market Report market research report
- ✔ Get in-depth analysis of Nuclear Medicine Therapeutics market size, growth, and forecasts.
- ✔ Understand Nuclear Medicine Therapeutics's regional dynamics and industry-specific trends.
- ✔ Identify potential applications, end-user demand, and growth segments in Nuclear Medicine Therapeutics
What is the Market Size & CAGR of Nuclear Medicine Therapeutics market in 2023?
Nuclear Medicine Therapeutics Industry Analysis
Nuclear Medicine Therapeutics Market Segmentation and Scope
Tell us your focus area and get a customized research report.
Nuclear Medicine Therapeutics Market Analysis Report by Region
Europe Nuclear Medicine Therapeutics Market Report:
The European market is projected to increase from USD 1.40 billion in 2023 to USD 2.75 billion by 2033, amid rising cancer incidences and the demand for targeted therapies. Strong regulatory frameworks and coordinated care models across European nations further enhance market growth.Asia Pacific Nuclear Medicine Therapeutics Market Report:
The Asia Pacific region's nuclear medicine therapeutics market is expected to expand from USD 0.84 billion in 2023 to USD 1.64 billion by 2033, driven by rising healthcare investments and improving diagnostic capabilities in countries like China and India. The region presents high growth potential due to an aging population and increasing chronic disease incidence.North America Nuclear Medicine Therapeutics Market Report:
North America will likely lead the market, growing from USD 1.63 billion in 2023 to USD 3.21 billion by 2033. Factors such as the early adoption of advanced healthcare technologies, extensive research initiatives, and a strong presence of key market players bolster this growth. The United States remains a significant contributor to the market.South America Nuclear Medicine Therapeutics Market Report:
In South America, the market will grow from USD 0.27 billion in 2023 to USD 0.53 billion by 2033. Expansion of healthcare infrastructure, improved access to advanced diagnostics, and awareness about nuclear medicine's efficacy are key growth drivers in this region, particularly in Brazil and Argentina.Middle East & Africa Nuclear Medicine Therapeutics Market Report:
In the Middle East and Africa, the market will rise from USD 0.36 billion in 2023 to USD 0.71 billion by 2033. Increased investment in healthcare infrastructure and the integration of innovative therapies in clinical practices are expected to drive market expansion in this region.Tell us your focus area and get a customized research report.
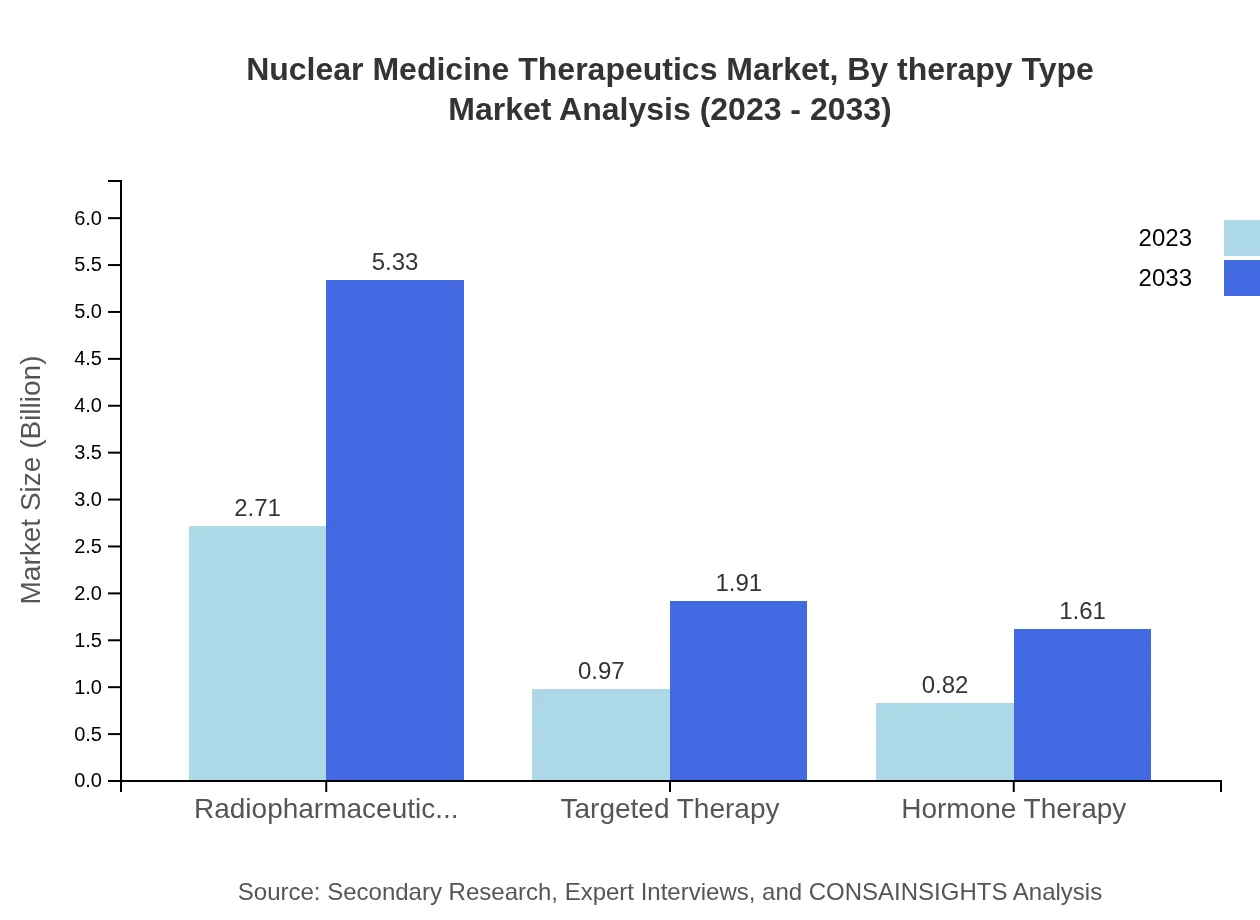
Nuclear Medicine Therapeutics Market Analysis By Therapy Type
The primary segments in therapy type include radiopharmaceuticals, targeted therapies, and hormone therapies. Radiopharmaceuticals dominate the market, representing approximately 60.3% share by 2023, with a growth forecast from USD 2.71 billion in 2023 to USD 5.33 billion in 2033. Targeted and hormone therapies are gaining momentum, reflecting advancements in personalized medicine and treatment efficacies.
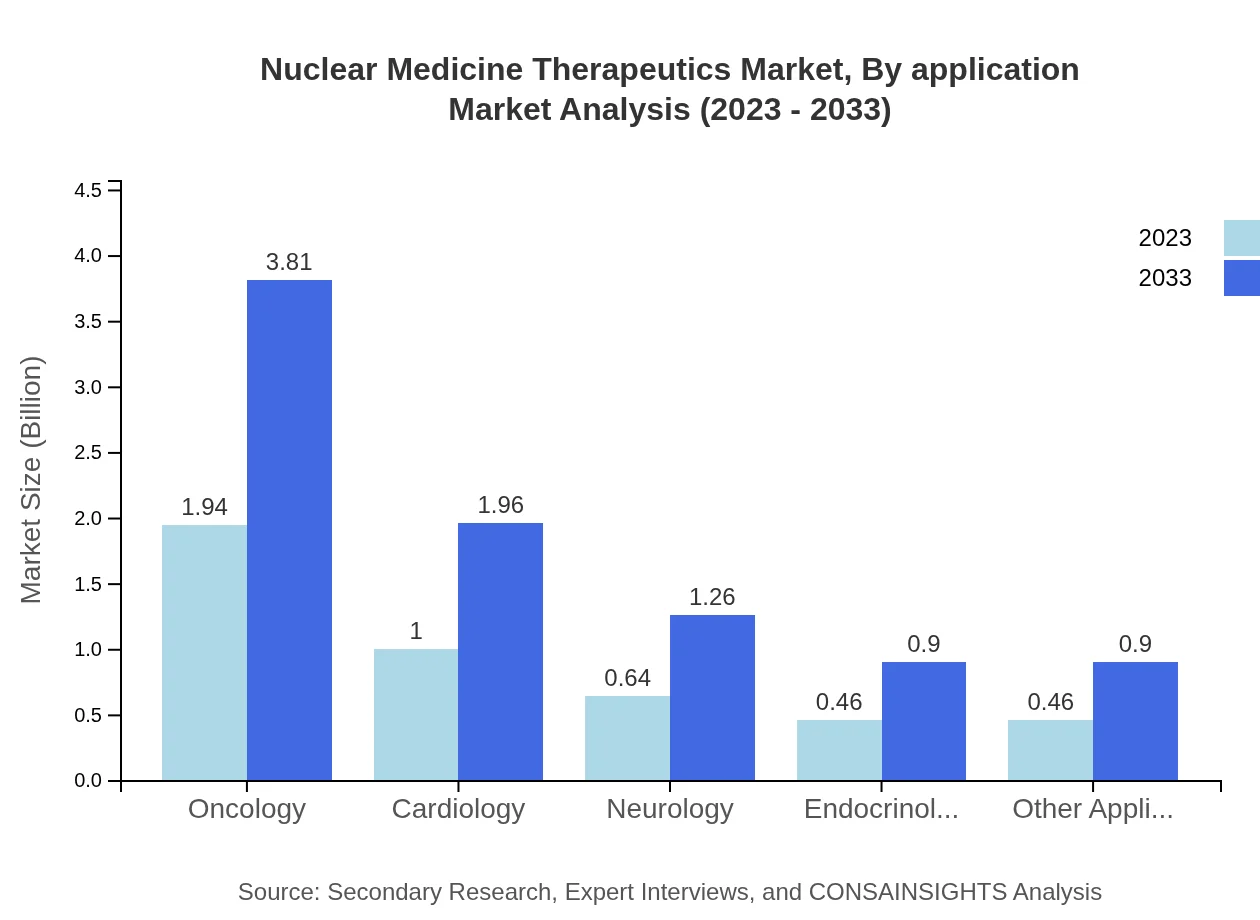
Nuclear Medicine Therapeutics Market Analysis By Application
Oncology leads as the most significant application, with a market size of USD 1.94 billion in 2023 and expected to reach USD 3.81 billion by 2033. Cardiology and neurology reflect notable growth, propelled by increased patient awareness and technological enhancements in diagnostics and therapies.
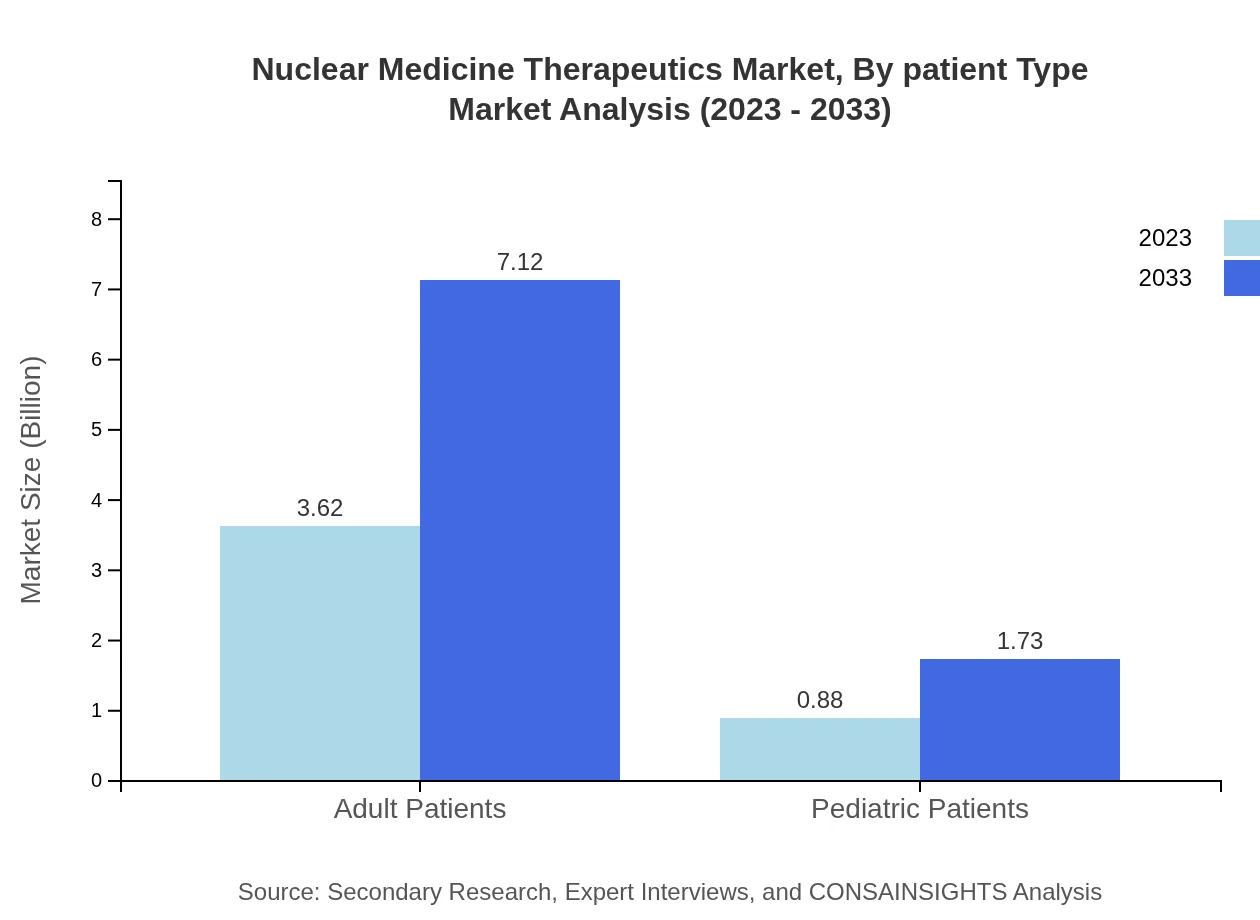
Nuclear Medicine Therapeutics Market Analysis By Patient Type
The adult patient segment is the largest, with a market size of USD 3.62 billion in 2023, projected to grow to USD 7.12 billion by 2033. Pediatric patient segments also show promise, increasing from USD 0.88 billion in 2023 to USD 1.73 billion owing to targeted healthcare initiatives.
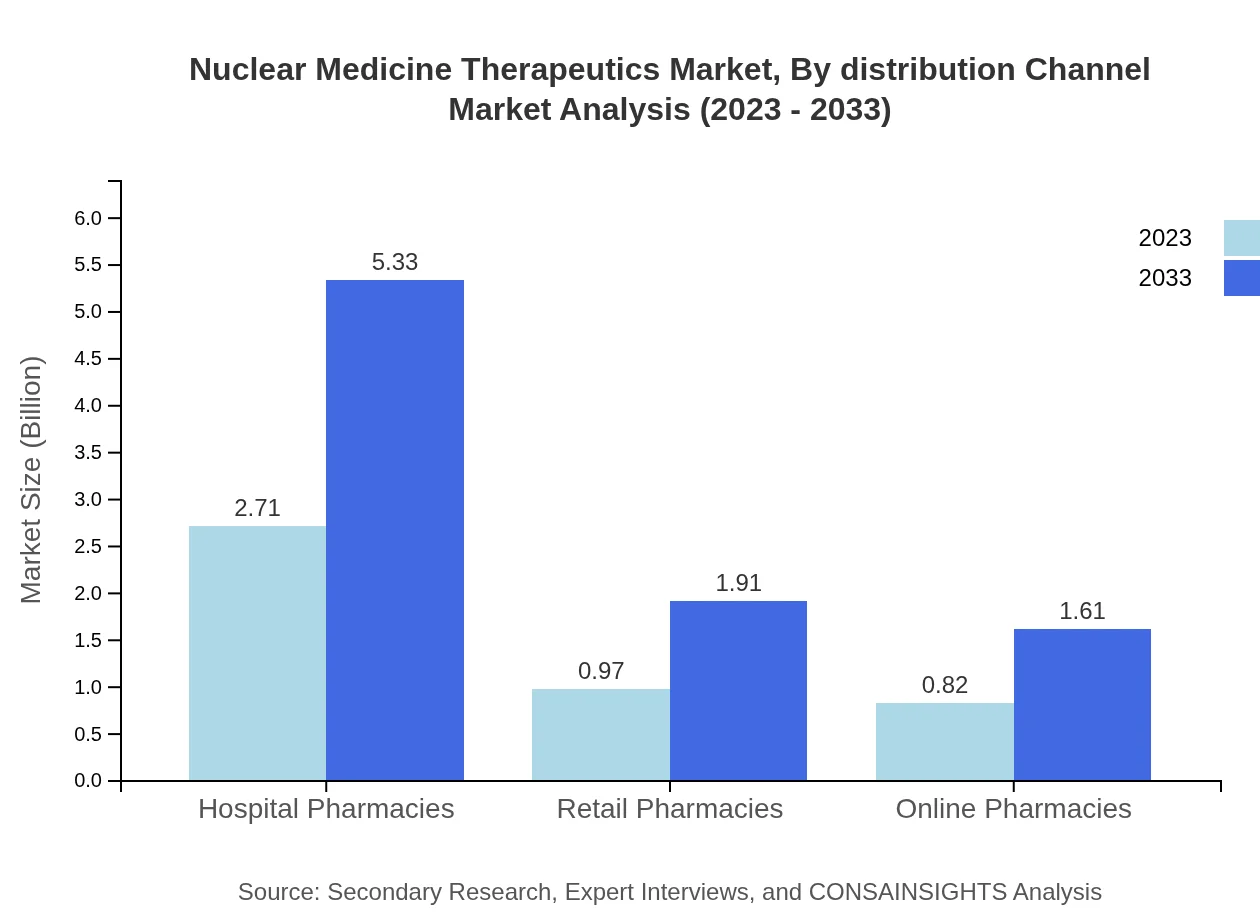
Nuclear Medicine Therapeutics Market Analysis By Distribution Channel
Hospital pharmacies hold a significant market share at 60.3%, with growth from USD 2.71 billion in 2023 to USD 5.33 billion by 2033. Retail and online pharmacies are also expanding, driven by increased patient access to medications via diverse platforms.
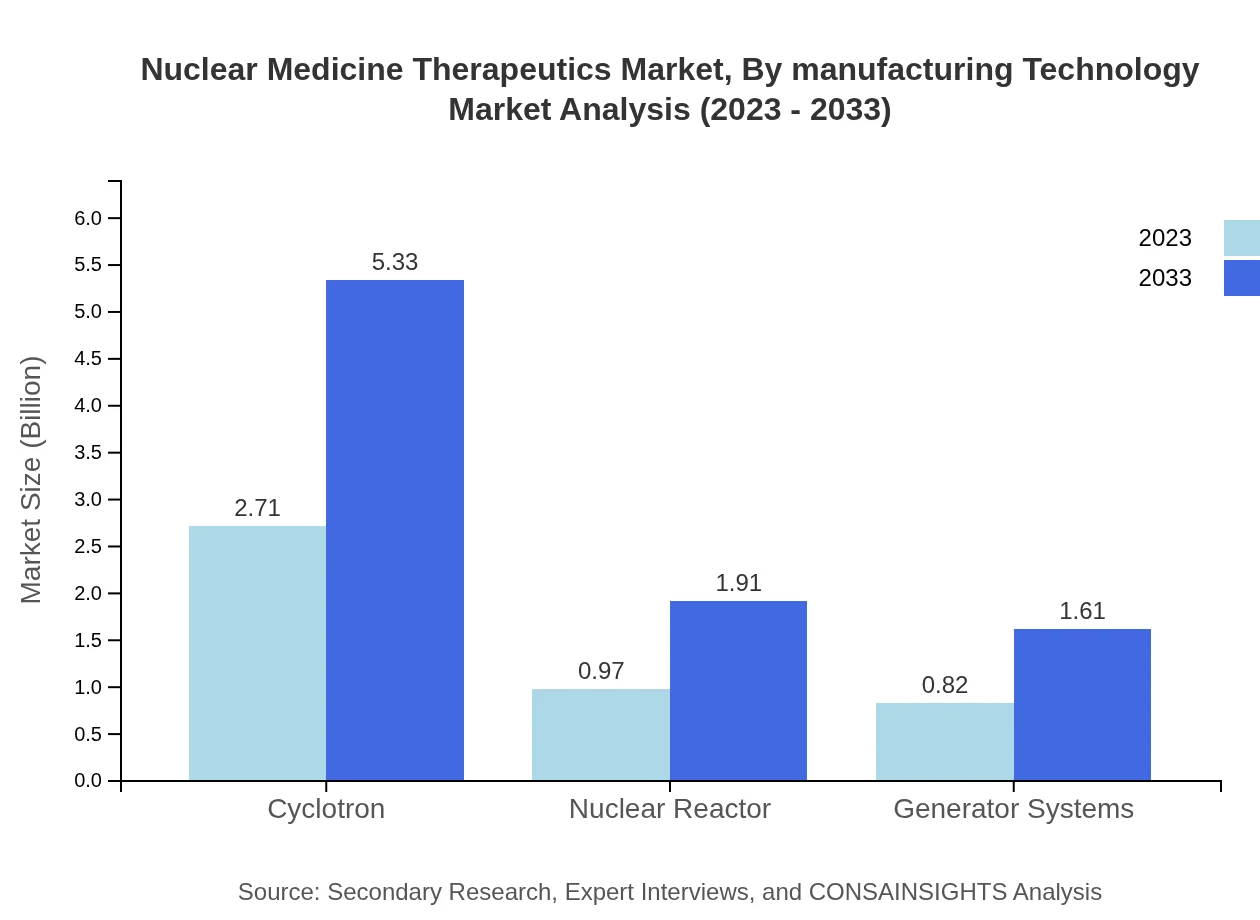
Nuclear Medicine Therapeutics Market Analysis By Manufacturing Technology
Manufacturing technologies for nuclear medicine therapeutics include cyclotrons, nuclear reactors, and generator systems. Cyclotrons lead with substantial market dominance, valued at USD 2.71 billion in 2023 and projected to reach USD 5.33 billion by 2033, representing technological innovation in radioisotope production.
Nuclear Medicine Therapeutics Market Trends and Future Forecast
Tell us your focus area and get a customized research report.
Global Market Leaders and Top Companies in Nuclear Medicine Therapeutics Industry
General Electric Healthcare:
A leading player in medical imaging and nuclear medicine, GE Healthcare specializes in innovative solutions for diagnostics and therapy management.Siemens Healthineers:
Siemens Healthineers focuses on advancing diagnostic imaging and therapy solutions, offering a comprehensive range of radiopharmaceuticals for oncology.Bayer AG:
Bayer is committed to healthcare innovations, particularly in radiopharmaceuticals, providing essential treatments in oncology and cardiology.Novartis:
Novartis is a global healthcare leader with a strong presence in nuclear medicine, pioneering advancements in targeted therapies and radiopharmaceuticals.Cardinal Health:
A key player in the nuclear medicine therapeutics market, Cardinal Health is renowned for its distribution of radiopharmaceuticals and innovative healthcare solutions.We're grateful to work with incredible clients.









FAQs
What is the market size of nuclear medicine therapeutics?
As of 2023, the global market size of nuclear medicine therapeutics is valued at approximately $4.5 billion, with a projected CAGR of 6.8%, indicating a strong growth trajectory through 2033.
What are the key market players or companies in this nuclear medicine therapeutics industry?
Key players in the nuclear medicine therapeutics sector include major pharmaceutical companies like Novartis, GE Healthcare, Siemens Healthineers, and Cardinal Health, all significantly contributing to innovation and market expansion.
What are the primary factors driving the growth in the nuclear medicine therapeutics industry?
Growth in the nuclear medicine therapeutics industry is driven by factors such as advancements in radiopharmaceutical technologies, increasing prevalence of cancer and cardiac diseases, and a growing aging population that necessitates effective treatment options.
Which region is the fastest Growing in the nuclear medicine therapeutics?
The North American region currently represents the largest market for nuclear medicine therapeutics, growing from $1.63 billion in 2023 to $3.21 billion by 2033, highlighting its rapid development and investment potential.
Does ConsaInsights provide customized market report data for the nuclear medicine therapeutics industry?
Yes, ConsaInsights offers customized market reports tailored to specific research needs in the nuclear medicine therapeutics industry, enabling stakeholders to gain insights that are directly applicable to their unique market situations.
What deliverables can I expect from this nuclear medicine therapeutics market research project?
Deliverables from a nuclear medicine therapeutics market research project include comprehensive reports, market forecasts, competitive analysis, strategic recommendations, and detailed segmentation data for informed decision-making.
What are the market trends of nuclear medicine therapeutics?
Current trends in the nuclear medicine therapeutics market include a shift towards personalized medicine, increased adoption of radiopharmaceuticals, and innovations in targeted therapies, all aimed at enhancing treatment efficacy and patient outcomes.