Nucleic Acid Amplification Testing Naat Market Report
Published Date: 31 January 2026 | Report Code: nucleic-acid-amplification-testing-naat
Nucleic Acid Amplification Testing Naat Market Size, Share, Industry Trends and Forecast to 2033
This report provides an extensive overview of the Nucleic Acid Amplification Testing (NAAT) market from 2023 to 2033, including market size, trends, regional analysis, technology insights, and a detailed look at industry dynamics.
| Metric | Value |
|---|---|
| Study Period | 2023 - 2033 |
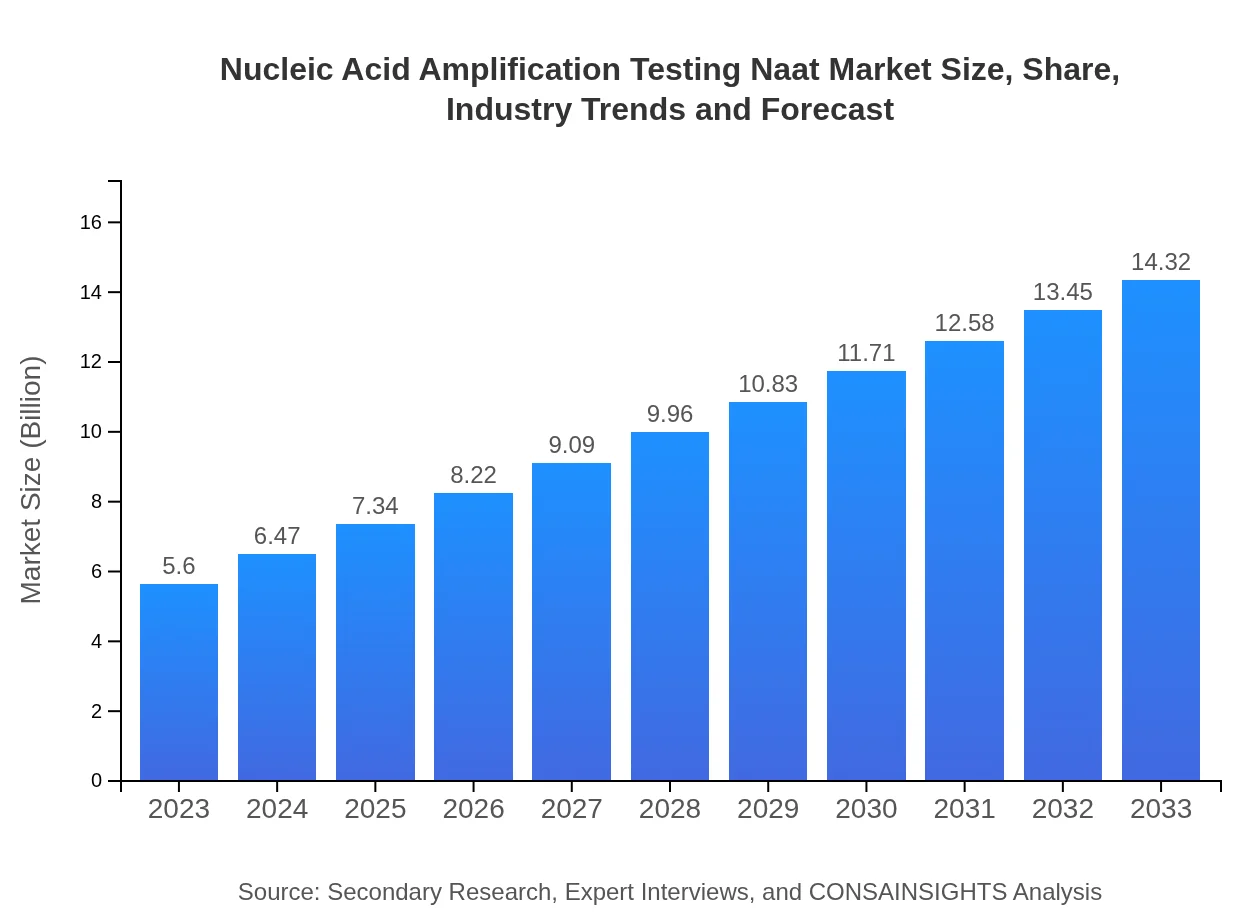
| 2023 Market Size | $5.60 Billion |
| CAGR (2023-2033) | 9.5% |
| 2033 Market Size | $14.32 Billion |
| Top Companies | Thermo Fisher Scientific, Roche Diagnostics, Abbott Laboratories, Qiagen , Cepheid |
| Last Modified Date | 31 January 2026 |
Nucleic Acid Amplification Testing Naat Market Overview
Customize Nucleic Acid Amplification Testing Naat Market Report market research report
- ✔ Get in-depth analysis of Nucleic Acid Amplification Testing Naat market size, growth, and forecasts.
- ✔ Understand Nucleic Acid Amplification Testing Naat's regional dynamics and industry-specific trends.
- ✔ Identify potential applications, end-user demand, and growth segments in Nucleic Acid Amplification Testing Naat
What is the Market Size & CAGR of Nucleic Acid Amplification Testing Naat market in {Year}?
Nucleic Acid Amplification Testing Naat Industry Analysis
Nucleic Acid Amplification Testing Naat Market Segmentation and Scope
Tell us your focus area and get a customized research report.
Nucleic Acid Amplification Testing Naat Market Analysis Report by Region
Europe Nucleic Acid Amplification Testing Naat Market Report:
In Europe, the market is forecasted to increase from $1.41 billion in 2023 to $3.60 billion by 2033, bolstered by regulatory support and technological advancements in diagnostic approaches.Asia Pacific Nucleic Acid Amplification Testing Naat Market Report:
The Asia Pacific NAAT market is projected to grow from $1.15 billion in 2023 to $2.94 billion by 2033, driven by increased healthcare spending, a large patient population, and the demand for advanced diagnostic methods.North America Nucleic Acid Amplification Testing Naat Market Report:
North America leads the market with a size of $2.09 billion in 2023, anticipated to grow to $5.34 billion by 2033. Factors include advanced healthcare infrastructure and high awareness of molecular diagnostics.South America Nucleic Acid Amplification Testing Naat Market Report:
The South American market is expected to expand from $0.35 billion in 2023 to $0.91 billion by 2033, spurred by rising healthcare investments and the increasing prevalence of infectious diseases requiring effective diagnostics.Middle East & Africa Nucleic Acid Amplification Testing Naat Market Report:
The Middle East and Africa market is set to grow from $0.60 billion in 2023 to $1.54 billion by 2033, aided by improving healthcare access and increased diagnostic initiatives across the region.Tell us your focus area and get a customized research report.
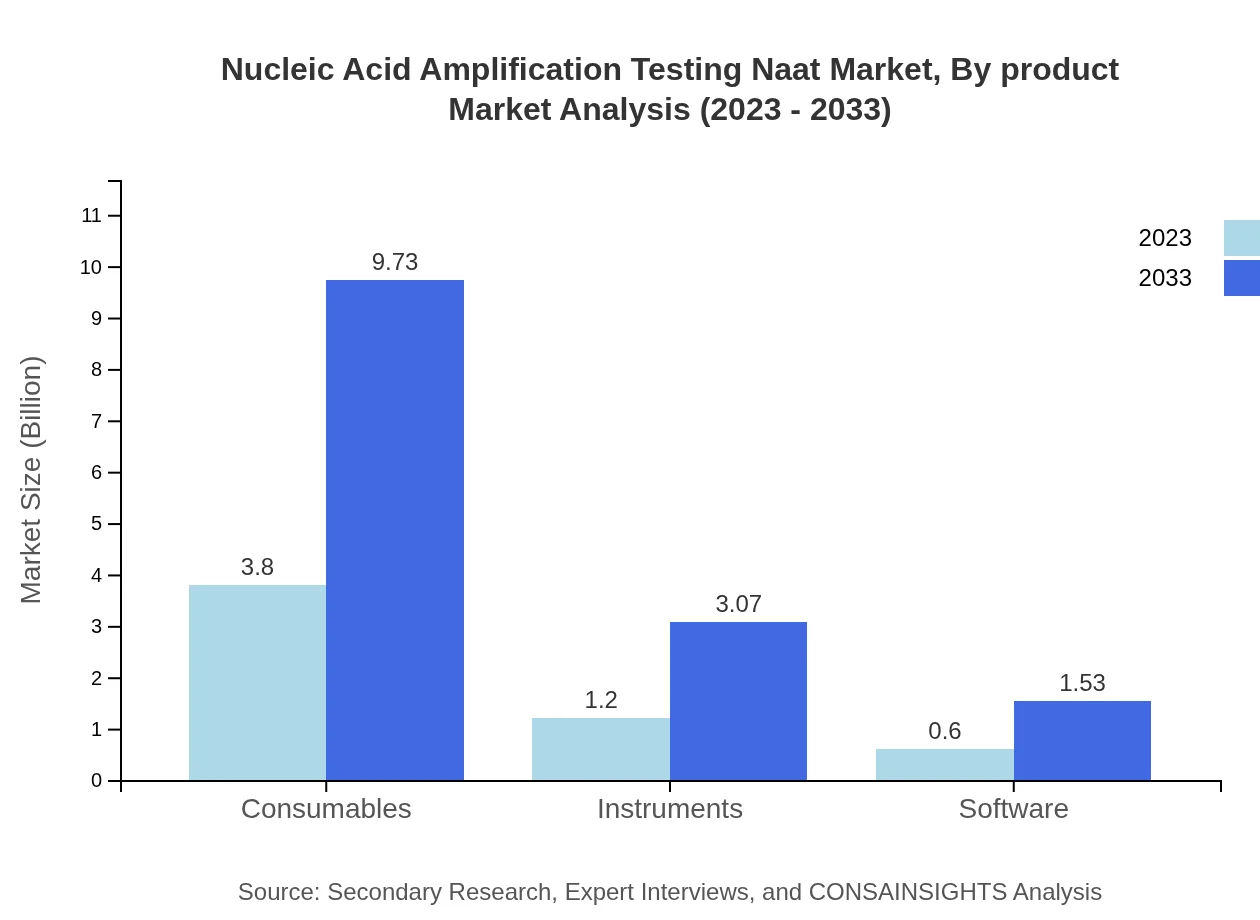
Nucleic Acid Amplification Testing Naat Market Analysis By Product
The consumables segment dominates the NAAT market, with a size of $3.80 billion in 2023 and expected to grow to $9.73 billion by 2033, accounting for 67.9% market share. The instruments segment is projected to grow from $1.20 billion to $3.07 billion and holds a share of 21.42%. Software solutions are anticipated to increase from $0.60 billion to $1.53 billion, maintaining 10.68% market share. These trends highlight the significant demand for consumables, driving overall market growth.
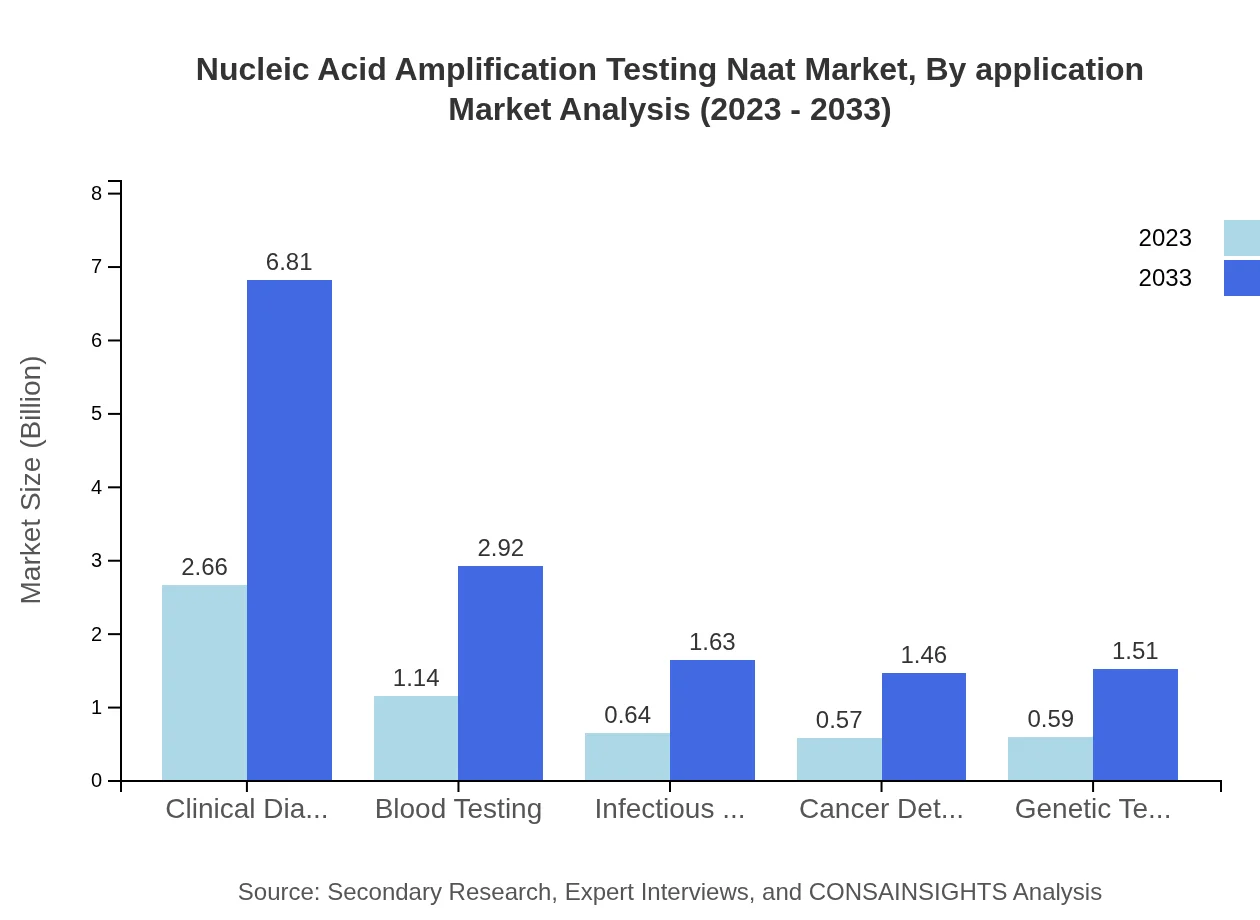
Nucleic Acid Amplification Testing Naat Market Analysis By Application
The clinical diagnostics segment is anticipated to dominate the application landscape, with a market size of $2.66 billion in 2023, projected to expand to $6.81 billion by 2033, maintaining the largest share at 47.51%. Blood testing and infectious disease applications represent substantial segments, with market sizes of $1.14 billion (20.42% share) and $0.64 billion (11.37% share) in 2023, respectively, indicating a strong focus on diagnostic applications.
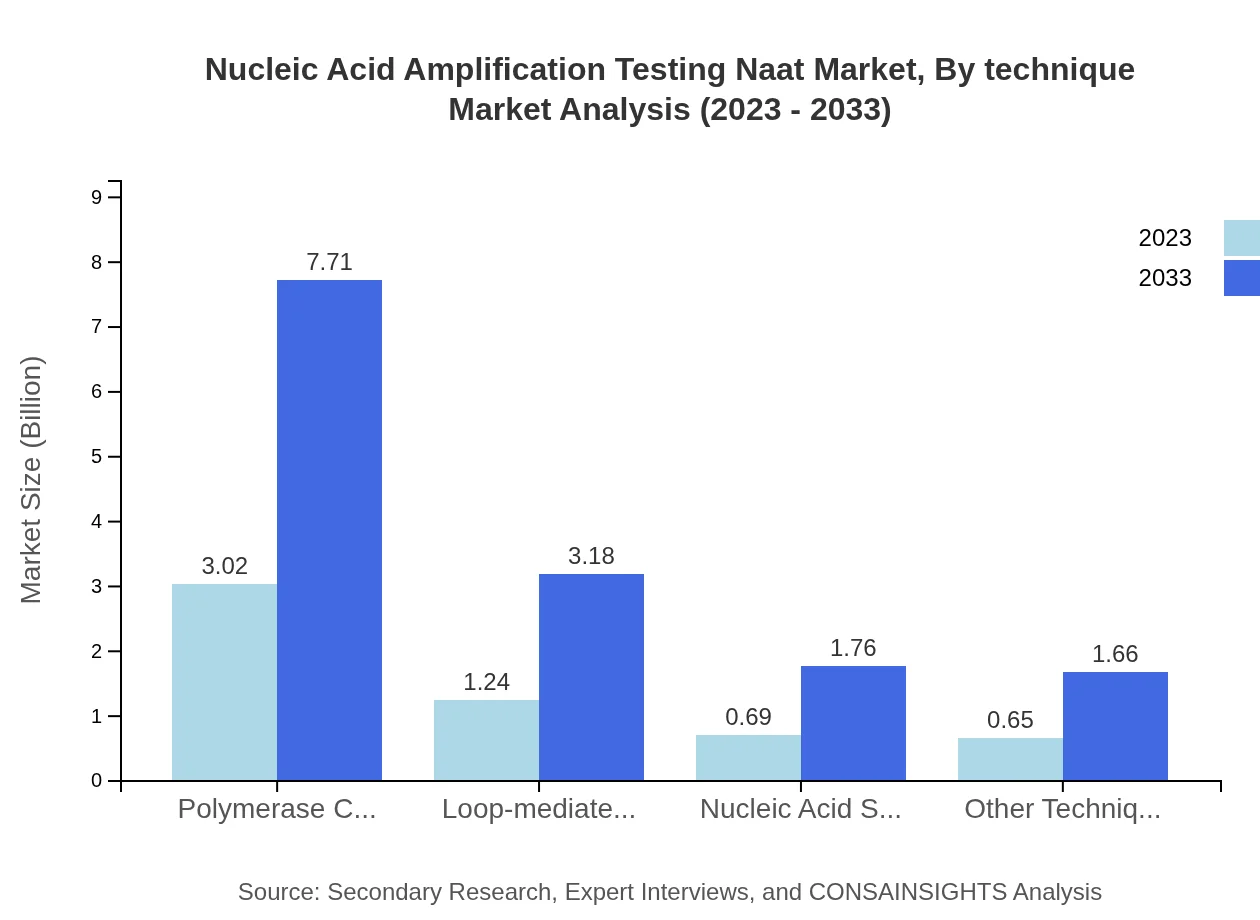
Nucleic Acid Amplification Testing Naat Market Analysis By Technique
The Polymerase Chain Reaction (PCR) segment leads in size at $3.02 billion in 2023, expected to grow to $7.71 billion by 2033, capturing a significant 53.86% market share. Loop-mediated Isothermal Amplification (LAMP) and Nucleic Acid Sequence-Based Amplification (NASBA) followed with forecasted market sizes of $1.24 billion and $0.69 billion respectively in 2023. These techniques are integral to the growth of the NAAT market, characterized by their efficiency and accuracy.
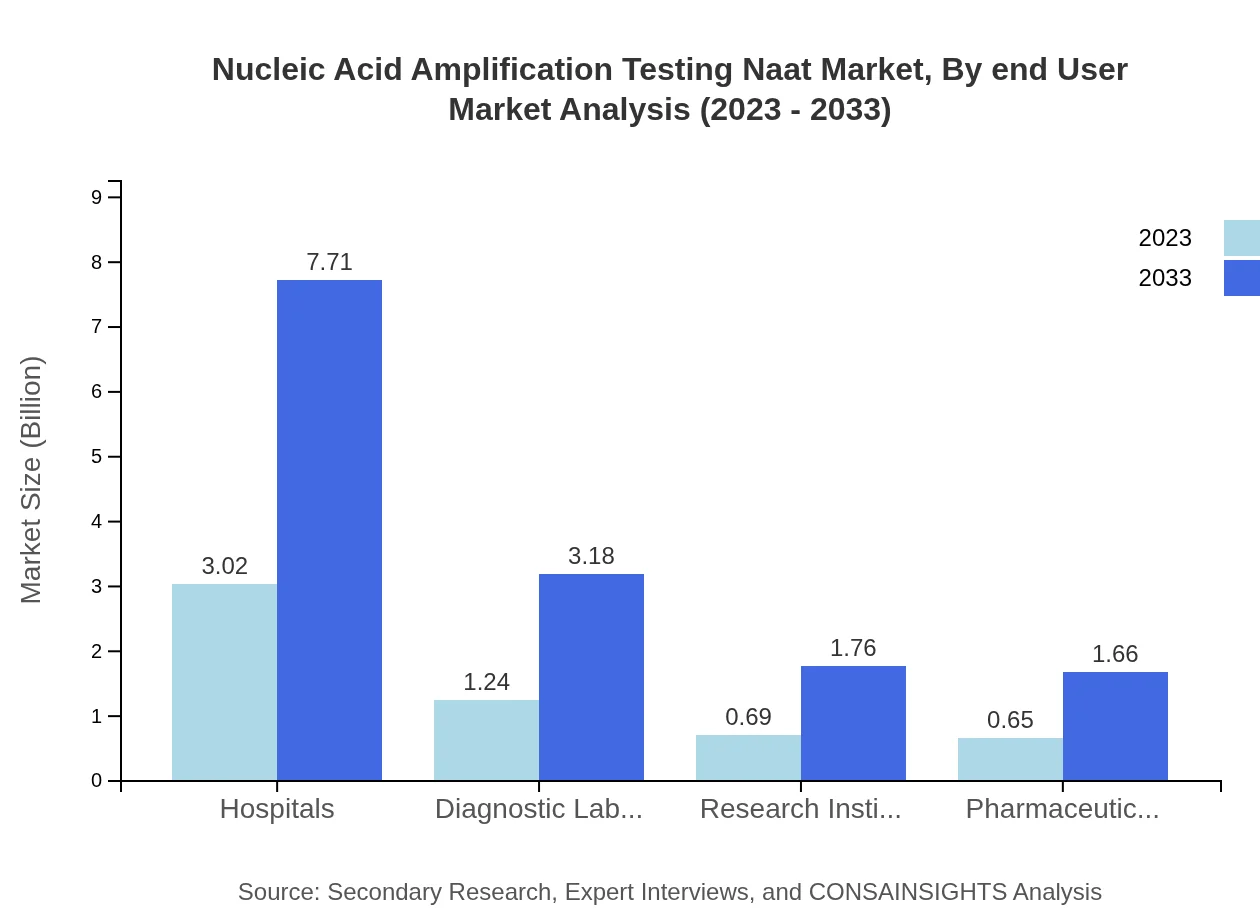
Nucleic Acid Amplification Testing Naat Market Analysis By End User
Hospitals remain the largest end-user segment, valued at $3.02 billion in 2023 and projected to reach $7.71 billion by 2033, holding a dominant share of 53.86%. Diagnostic laboratories and research institutes are also prominent, with forecasted sizes of $1.24 billion (22.23% share) and $0.69 billion (12.31% share) in 2023, emphasizing the critical role of hospital and laboratory settings in NAAT applications.
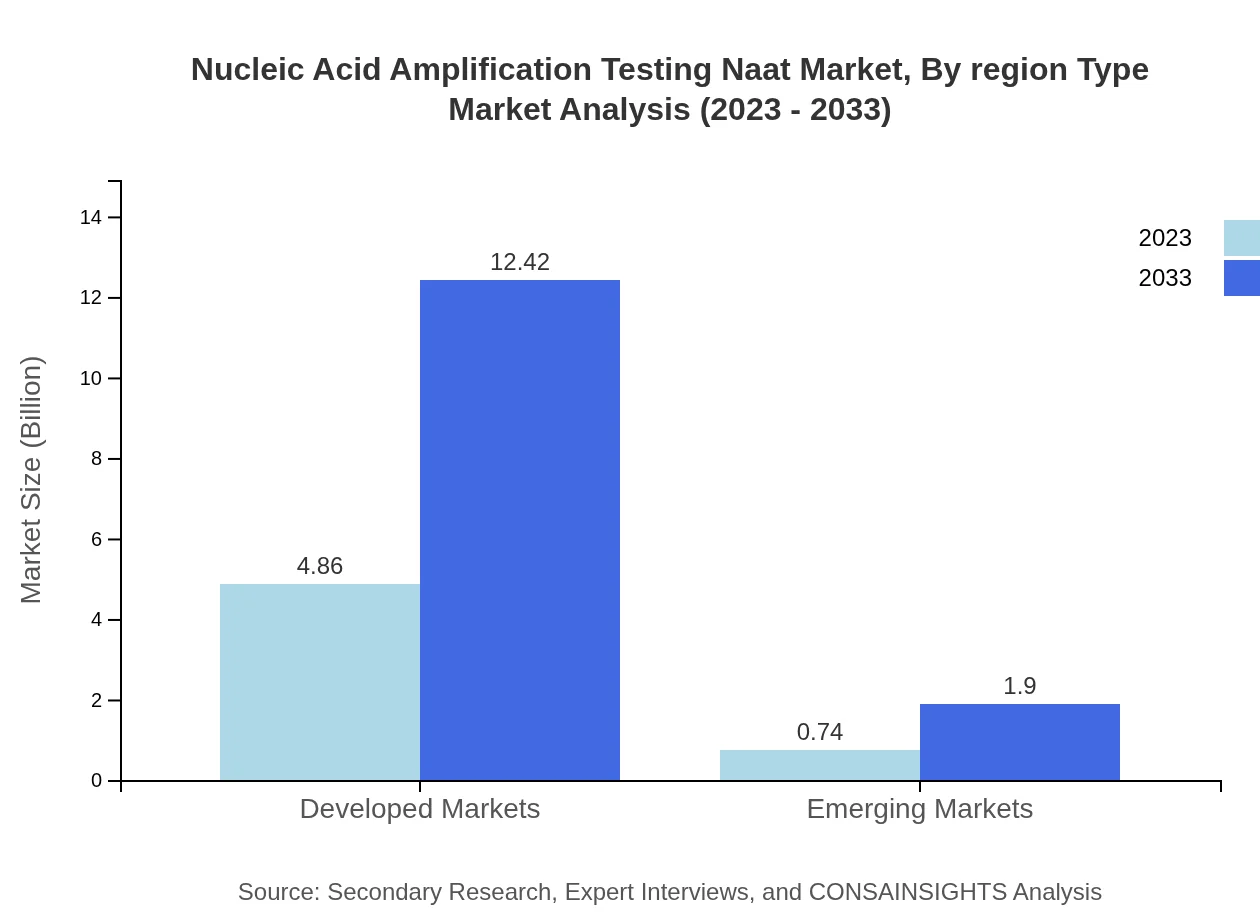
Nucleic Acid Amplification Testing Naat Market Analysis By Region Type
The NAAT market is experiencing varied growth patterns across regions, with developed markets growing from $4.86 billion in 2023 to $12.42 billion by 2033, representing an 86.72% market share. In contrast, emerging markets are anticipated to grow from $0.74 billion to $1.90 billion, capturing a 13.28% share. This divergence reflects the broader adoption of NAAT technologies in developed regions compared to emerging markets.
Nucleic Acid Amplification Testing Naat Market Trends and Future Forecast
Tell us your focus area and get a customized research report.
Global Market Leaders and Top Companies in Nucleic Acid Amplification Testing Naat Industry
Thermo Fisher Scientific:
Thermo Fisher Scientific is a global leader in serving science, providing a wide range of NAAT solutions and optimizing molecular diagnostics technologies across various applications.Roche Diagnostics:
Roche is a pioneer in the field of molecular diagnostics, offering an extensive portfolio of NAAT products that enhance patient testing and improve clinical outcomes.Abbott Laboratories:
Abbott is recognized for its innovative diagnostics, including advanced NAAT systems that are crucial for rapid and accurate detection of infectious diseases.Qiagen :
Qiagen offers cutting-edge molecular testing solutions, specializing in NAAT technologies that support laboratories in achieving reliable and efficient testing processes.Cepheid:
Cepheid is renowned for its automated molecular testing solutions, providing quick and precise NAAT systems for a variety of clinical applications.We're grateful to work with incredible clients.









FAQs
What is the market size of nucleic Acid Amplification Testing Naat?
The global nucleic acid amplification testing market size reached approximately $5.6 billion in 2023. It is projected to grow at a robust CAGR of 9.5%, reaching significant heights by 2033.
What are the key market players or companies in this nucleic Acid Amplification Testing Naat industry?
Key players in the nucleic acid amplification testing market include Roche, Thermo Fisher Scientific, Abbott Laboratories, Qiagen, and Bio-Rad Laboratories. These companies are pivotal in developing advanced NAAT technologies and capturing significant market shares.
What are the primary factors driving the growth in the nucleic Acid Amplification Testing Naat industry?
Key drivers of growth in the NAAT industry include rising demand for rapid diagnostics, increased incidence of infectious diseases, advancements in molecular biology techniques, and growing investments in pharmaceutical research and development.
Which region is the fastest Growing in the nucleic Acid Amplification Testing Naat?
The fastest-growing region for nucleic acid amplification testing is North America, with a market size of $2.09 billion in 2023 projected to reach $5.34 billion by 2033, fueled by technological advancements and high healthcare expenditure.
Does ConsaInsights provide customized market report data for the nucleic Acid Amplification Testing Naat industry?
Yes, ConsaInsights offers customized market report data for the nucleic acid amplification testing industry. Reports can be tailored to meet specific client needs, including unique parameters and regional insights.
What deliverables can I expect from this nucleic Acid Amplification Testing Naat market research project?
Deliverables from the NAAT market research project include comprehensive market analysis, competitive landscape evaluation, forecasts through 2033, and insights on trends, challenges, and opportunities across various segments.
What are the market trends of nucleic Acid Amplification Testing Naat?
Trends in the nucleic acid amplification testing market include increased automation in laboratories, expansion into emerging markets, integration of AI for diagnostic accuracy, and a focus on point-of-care testing solutions.