Obstetrics Devices Market Report
Published Date: 31 January 2026 | Report Code: obstetrics-devices
Obstetrics Devices Market Size, Share, Industry Trends and Forecast to 2033
This report provides an in-depth analysis of the Obstetrics Devices market, covering key trends, market segmentation, and forecasts from 2023 to 2033, alongside insights on competitive landscape and technological advancements.
| Metric | Value |
|---|---|
| Study Period | 2023 - 2033 |
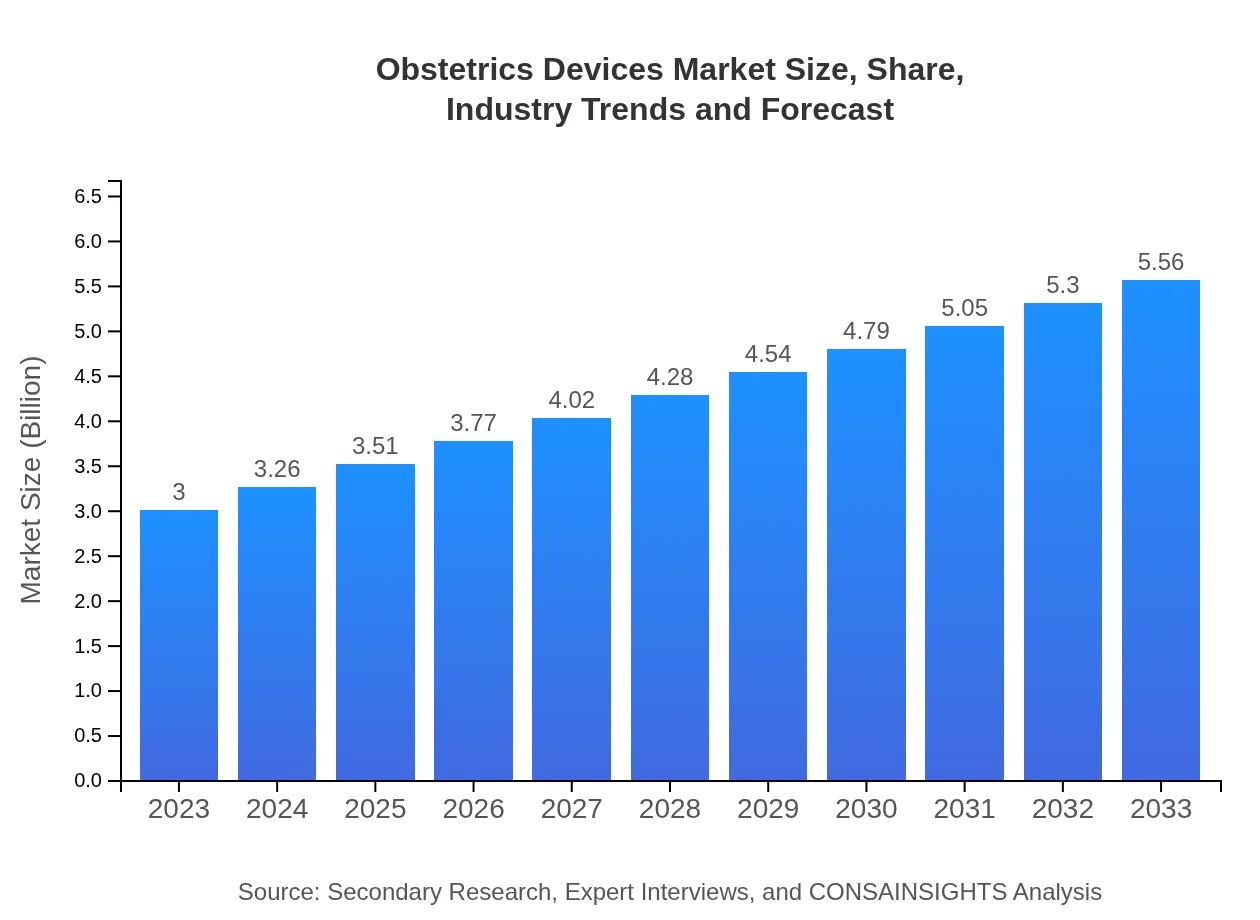
| 2023 Market Size | $3.00 Billion |
| CAGR (2023-2033) | 6.2% |
| 2033 Market Size | $5.56 Billion |
| Top Companies | GE Healthcare, Siemens Healthineers, Philips Healthcare, Medtronic |
| Last Modified Date | 31 January 2026 |
Obstetrics Devices Market Overview
Customize Obstetrics Devices Market Report market research report
- ✔ Get in-depth analysis of Obstetrics Devices market size, growth, and forecasts.
- ✔ Understand Obstetrics Devices's regional dynamics and industry-specific trends.
- ✔ Identify potential applications, end-user demand, and growth segments in Obstetrics Devices
What is the Market Size & CAGR of Obstetrics Devices market in 2023?
Obstetrics Devices Industry Analysis
Obstetrics Devices Market Segmentation and Scope
Tell us your focus area and get a customized research report.
Obstetrics Devices Market Analysis Report by Region
Europe Obstetrics Devices Market Report:
The European Obstetrics Devices market is anticipated to rise from $0.97 billion in 2023 to $1.79 billion by 2033. Factors such as stringent regulations ensuring high-quality medical devices, alongside rising health awareness and technological improvements, are fueling growth in this region. Countries like Germany, the UK, and France lead the market.Asia Pacific Obstetrics Devices Market Report:
The Obstetrics Devices market in Asia Pacific is projected to grow from $0.52 billion in 2023 to $0.96 billion by 2033. Factors driving this growth include rising maternal health awareness, increasing access to healthcare facilities, and the growing number of childbirths in countries like India and China. Additionally, government investments in healthcare infrastructure are expected to bolster the market further.North America Obstetrics Devices Market Report:
The North American market, valued at $1.09 billion in 2023, is projected to reach $2.02 billion by 2033. The growth is propelled by advanced healthcare systems, high disposable income, and ongoing innovations in obstetrics technology. The U.S. holds the largest market share, fueled by a significant focus on research and development in maternal health technologies.South America Obstetrics Devices Market Report:
In South America, the market is expected to expand from $0.18 billion in 2023 to $0.34 billion by 2033. The growth is primarily driven by enhanced healthcare access and rising maternal care investments. Key countries like Brazil and Argentina are increasingly focusing on improving maternal health services, contributing to this market expansion.Middle East & Africa Obstetrics Devices Market Report:
The market in the Middle East and Africa is projected to grow from $0.24 billion in 2023 to $0.44 billion by 2033. Increased healthcare investments and the growing emphasis on maternal healthcare in countries such as the UAE and South Africa are driving the expansion of this market.Tell us your focus area and get a customized research report.
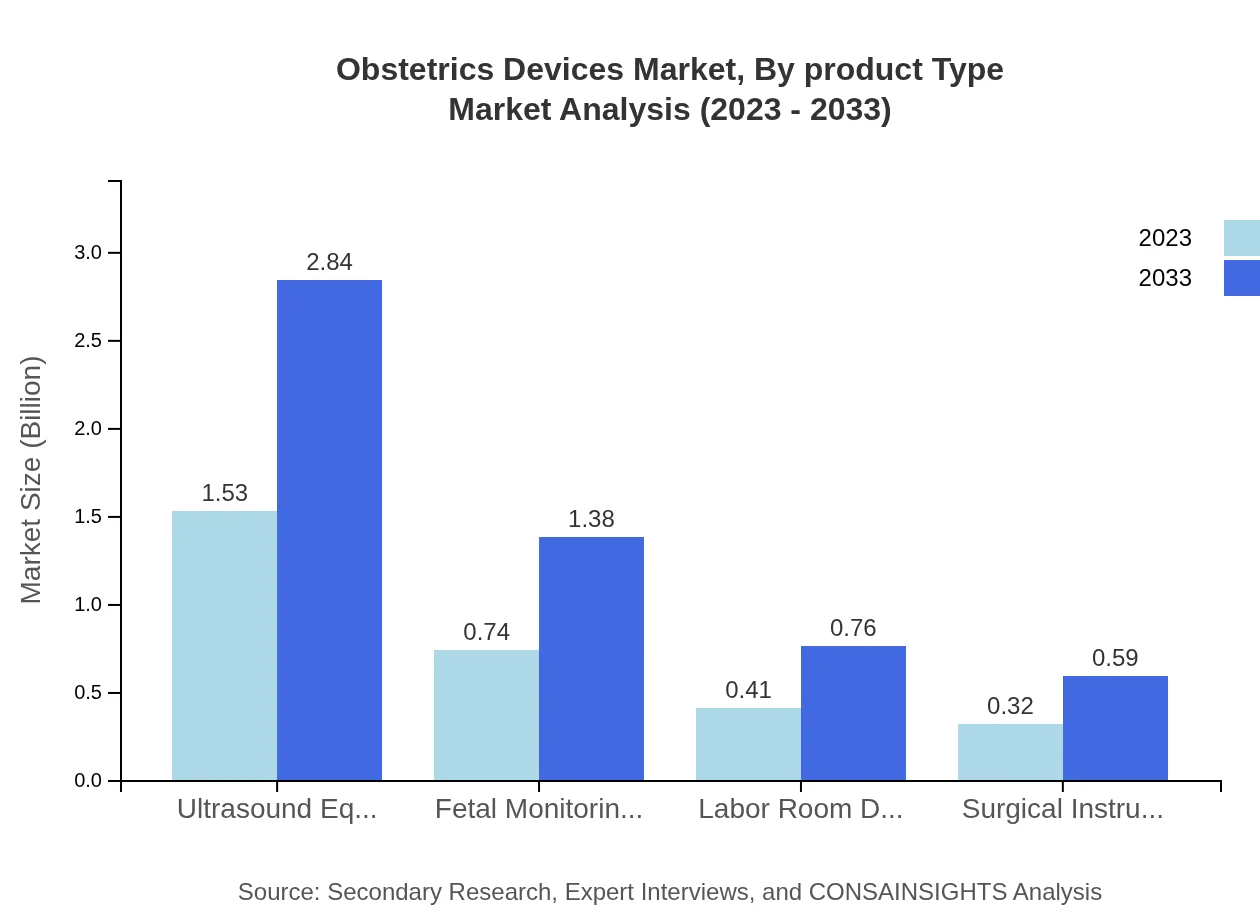
Obstetrics Devices Market Analysis By Product Type
The Obstetrics Devices market by product type includes categories such as fetal monitoring devices, surgical instruments, ultrasound equipment, and prenatal care devices. Fetal monitoring devices dominate the market due to their critical role in monitoring fetal health during pregnancy, constituting approximately 51.08% of the market in 2023 and growing steadily. As the industry progresses, digital technologies are increasingly becoming significant contributors, emphasizing the blend of traditional practices with modern innovations.
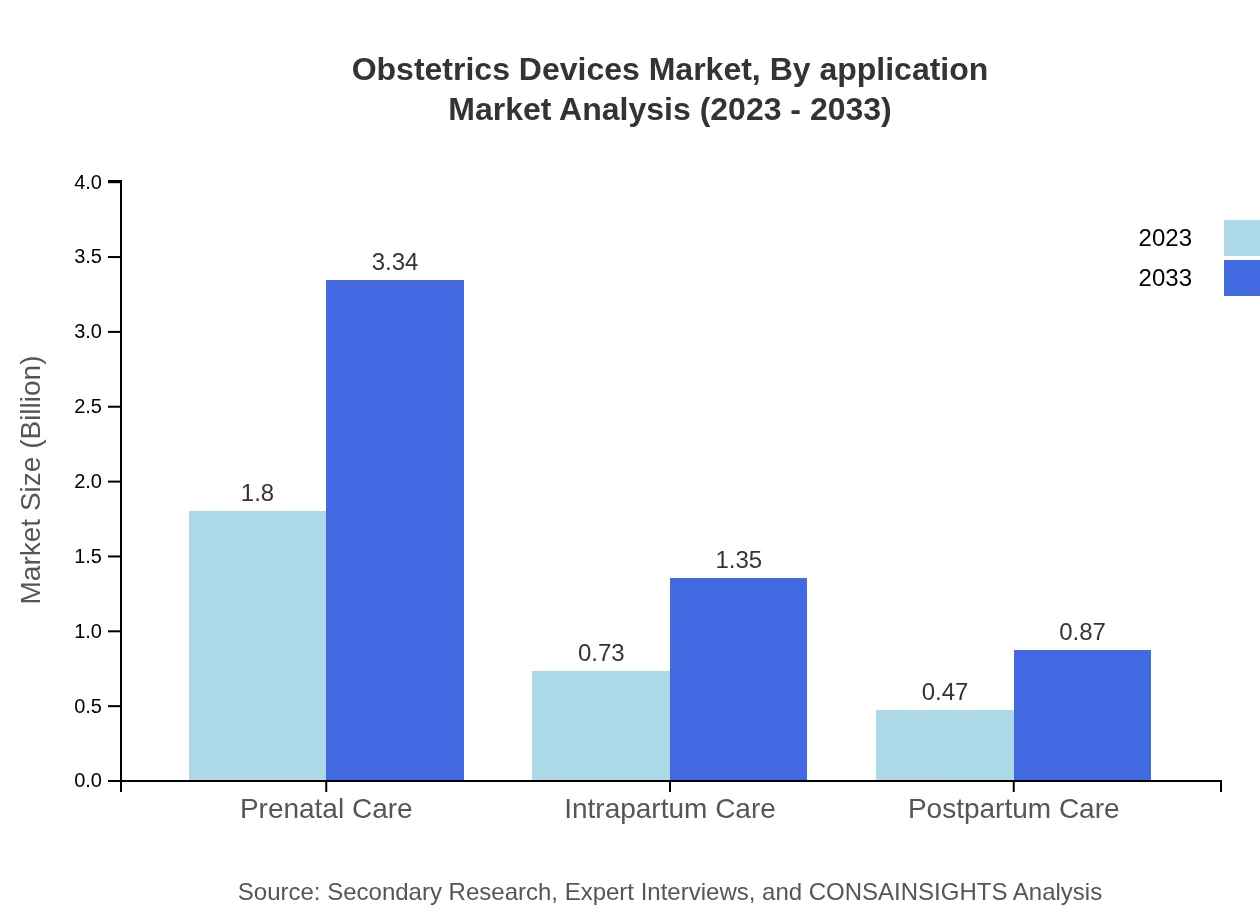
Obstetrics Devices Market Analysis By Application
The market analysis by application reveals prenatal care, intrapartum care, and postpartum care as key segments. Prenatal care accounts for 60.07% of the market share in 2023, highlighting its importance in ensuring the health of both mother and child. The increasing focus on comprehensive maternity services is anticipated to further bolster these segments, as healthcare providers prioritize holistic maternal care.
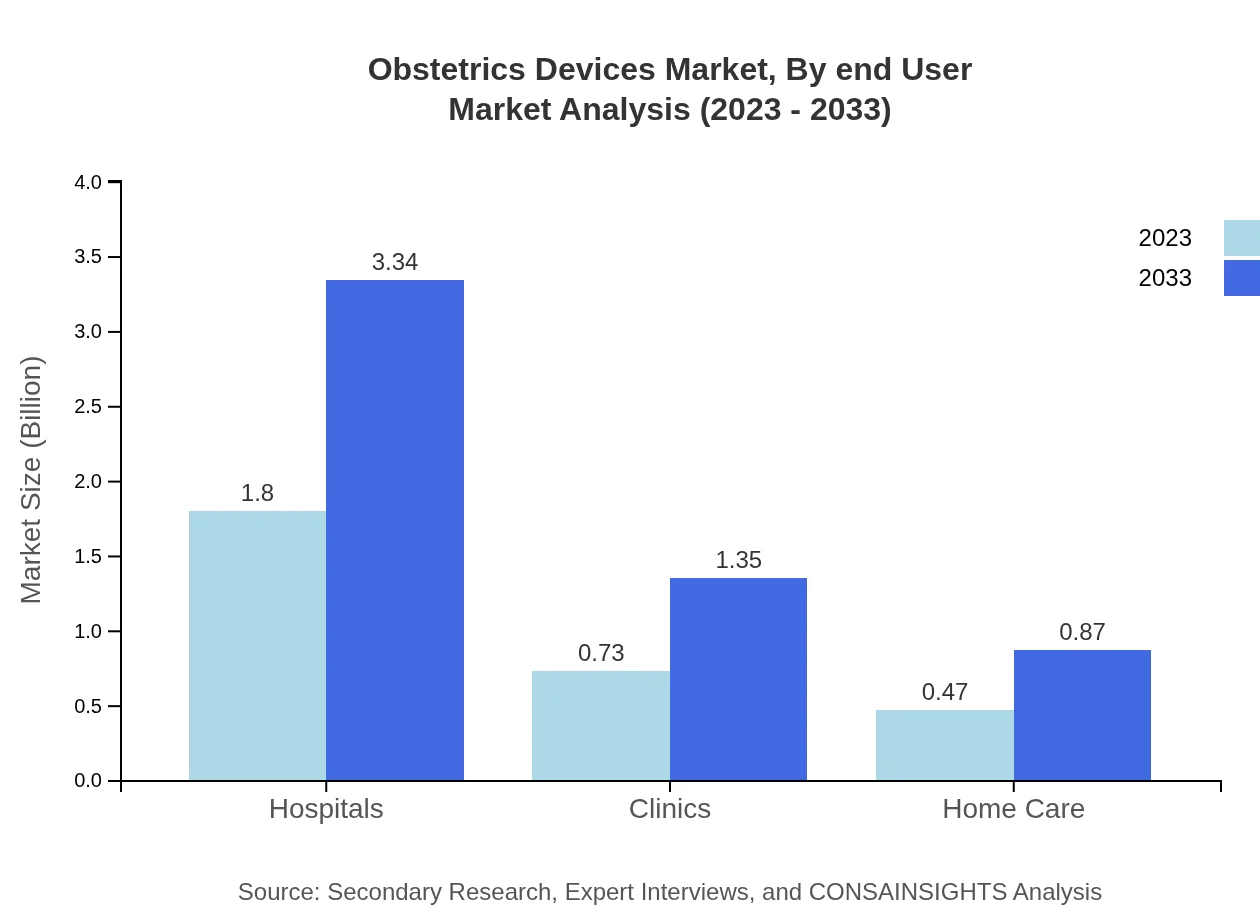
Obstetrics Devices Market Analysis By End User
The segmentation by end-user reveals hospitals as the primary users of obstetrics devices, accounting for over 60% of the market share. Clinics and home care facilities are also significant contributors. The hospital segment is expected to grow from $1.80 billion in 2023 to $3.34 billion by 2033, driven by the demand for specialized maternal care services and advanced medical technologies.
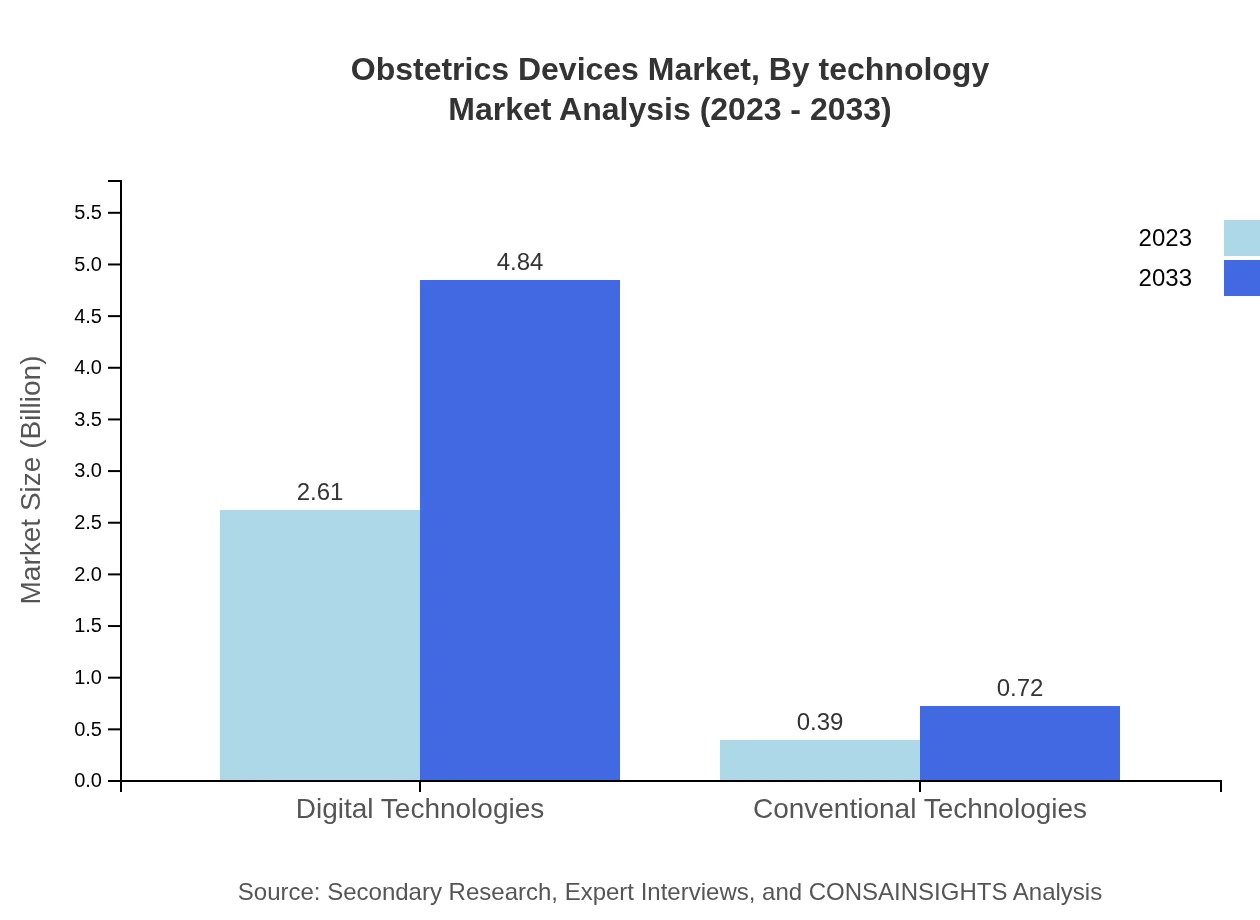
Obstetrics Devices Market Analysis By Technology
The Obstetrics Devices market is increasingly influenced by technological advancements. Digital technologies dominate with a market share of 87.01% in 2023, showcasing the growing reliance on innovation to enhance patient care and operational efficiency. As the healthcare space continues to adopt electronic health records and telehealth solutions, the market is poised for further technological integration.
Obstetrics Devices Market Trends and Future Forecast
Tell us your focus area and get a customized research report.
Global Market Leaders and Top Companies in Obstetrics Devices Industry
GE Healthcare:
A leading global provider of medical imaging and monitoring solutions, known for its innovative ultrasound systems and fetal monitoring technologies.Siemens Healthineers:
Renowned for its cutting-edge medical devices, particularly advanced imaging and laboratory diagnostics equipment that enhance maternal care.Philips Healthcare:
Specializes in various healthcare solutions, including high-quality ultrasound devices and software for enhanced patient monitoring during pregnancy.Medtronic :
A major player in the medical technology space, offering a range of devices and solutions for maternal-fetal medicine and surgical interventions.We're grateful to work with incredible clients.









FAQs
What is the market size of obstetrics Devices?
The obstetrics devices market is currently valued at approximately $3 billion in 2023 and is projected to grow at a CAGR of 6.2%, reaching an estimated market size in the coming years as healthcare demands evolve.
What are the key market players or companies in this obstetrics Devices industry?
Key players in the obstetrics devices market include large medical device manufacturers and technology companies focusing on innovation, safety, and efficiency in obstetric care, featuring both established companies and emerging startups.
What are the primary factors driving the growth in the obstetrics Devices industry?
Growth in the obstetrics devices market is primarily driven by technological advancements, rising birth rates, increasing awareness and demand for maternal healthcare, and a greater emphasis on prenatal and postpartum care among healthcare providers.
Which region is the fastest Growing in the obstetrics Devices?
North America is the fastest-growing region in the obstetrics devices market, with a market size projected to increase from $1.09 billion in 2023 to $2.02 billion by 2033, catalyzing innovation and access to advanced obstetric care.
Does ConsaInsights provide customized market report data for the obstetrics Devices industry?
Yes, ConsaInsights offers customized market reports for the obstetrics-devices industry, providing tailored data and insights based on specific needs and focus areas, ensuring clients receive relevant and actionable market intelligence.
What deliverables can I expect from this obstetrics Devices market research project?
Deliverables from the obstetrics devices market research project include a comprehensive report with market size data, trends, competitive analysis, forecasts, and region or segment-level insights to support informed business decisions.
What are the market trends of obstetrics Devices?
Current trends in the obstetrics devices market include increasing adoption of digital health technologies, enhanced focus on patient-centered care, integration of AI and machine learning in obstetric practices, and an expanding demand for home care solutions.