Ocular Drug Delivery Market Report
Published Date: 31 January 2026 | Report Code: ocular-drug-delivery
Ocular Drug Delivery Market Size, Share, Industry Trends and Forecast to 2033
This report provides an extensive analysis of the Ocular Drug Delivery market, including market size, segmentation, regional insights, and future forecasts from 2023 to 2033, highlighting trends and growth opportunities.
| Metric | Value |
|---|---|
| Study Period | 2023 - 2033 |
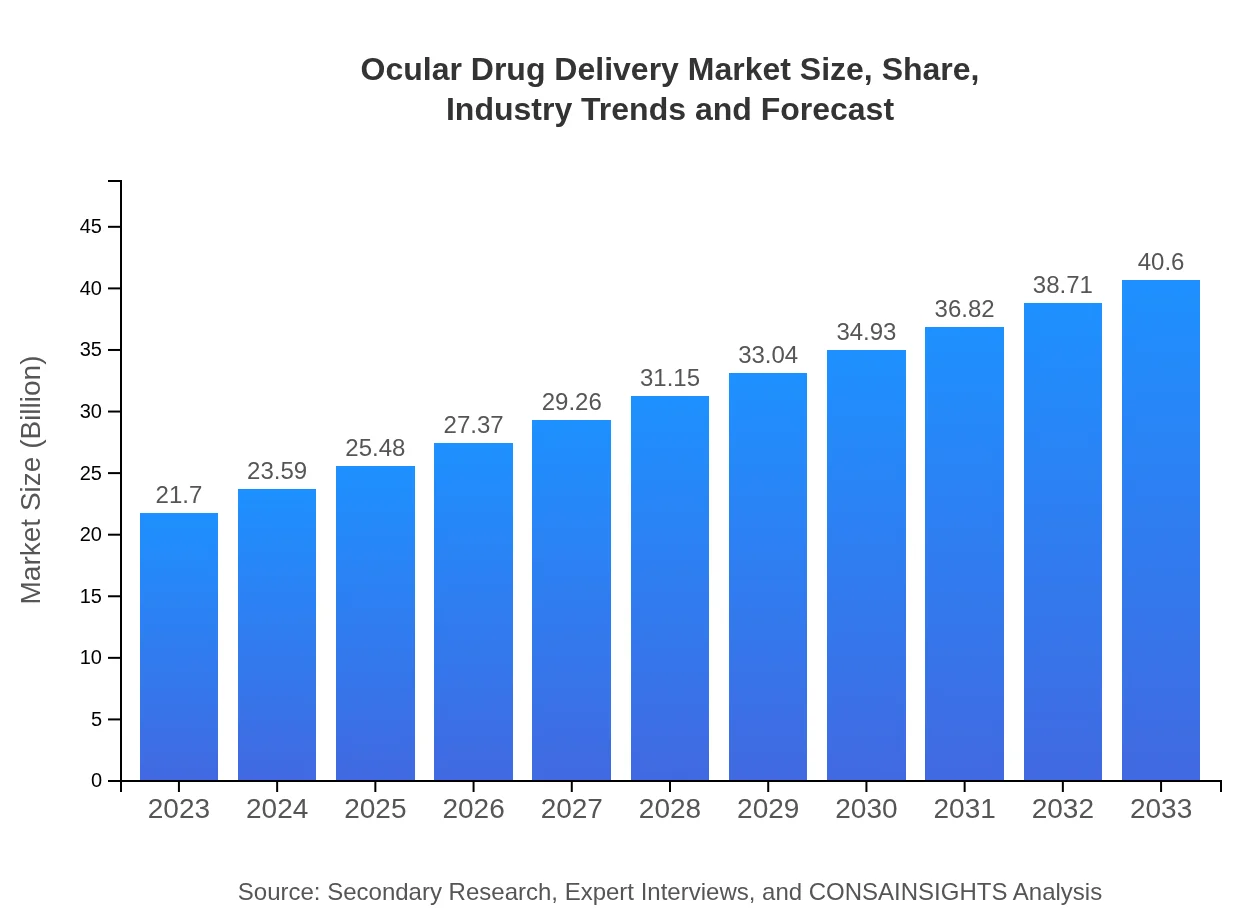
| 2023 Market Size | $21.70 Billion |
| CAGR (2023-2033) | 6.3% |
| 2033 Market Size | $40.60 Billion |
| Top Companies | Allergan, Novartis, Bausch + Lomb, Regeneron Pharmaceuticals |
| Last Modified Date | 31 January 2026 |
Ocular Drug Delivery Market Overview
Customize Ocular Drug Delivery Market Report market research report
- ✔ Get in-depth analysis of Ocular Drug Delivery market size, growth, and forecasts.
- ✔ Understand Ocular Drug Delivery's regional dynamics and industry-specific trends.
- ✔ Identify potential applications, end-user demand, and growth segments in Ocular Drug Delivery
What is the Market Size & CAGR of Ocular Drug Delivery market in 2023?
Ocular Drug Delivery Industry Analysis
Ocular Drug Delivery Market Segmentation and Scope
Tell us your focus area and get a customized research report.
Ocular Drug Delivery Market Analysis Report by Region
Europe Ocular Drug Delivery Market Report:
The European market for ocular drug delivery is forecasted to grow from $6.55 billion in 2023 to $12.26 billion by 2033. Factors such as an increase in the aging population, partnerships between industry players for innovation, and favorable regulatory frameworks enhance market conditions within the region.Asia Pacific Ocular Drug Delivery Market Report:
In the Asia Pacific region, the ocular drug delivery market is expected to grow from $3.75 billion in 2023 to $7.02 billion by 2033, driven by increasing healthcare expenditure, a rising aging population, and the prevalence of ocular diseases. The growing number of research and development initiatives in countries like China and India is boosting market conditions, offering more advanced ocular therapies.North America Ocular Drug Delivery Market Report:
North America currently leads the ocular drug delivery market with a valuation of $8.29 billion in 2023, set to grow to $15.52 billion by 2033. This growth is attributed to advanced healthcare systems, high demand for innovative therapies, and significant investments in ocular drug delivery research. The U.S. remains a focal point for numerous clinical trials and product launches.South America Ocular Drug Delivery Market Report:
The South American ocular drug delivery market, valued at $1.53 billion in 2023, is projected to reach $2.87 billion by 2033. Key factors include improving healthcare infrastructure and heightened awareness of ocular diseases leading to increased healthcare spending. Brazil stands out as a significant contributor to this growth.Middle East & Africa Ocular Drug Delivery Market Report:
The Middle East and Africa market is projected to increase from $1.57 billion in 2023 to $2.94 billion by 2033. Market growth is driven by an increasing focus on healthcare reforms, improved access to eye care facilities, and rising awareness regarding ocular health among the population.Tell us your focus area and get a customized research report.
Ocular Drug Delivery Market Analysis By Product Type
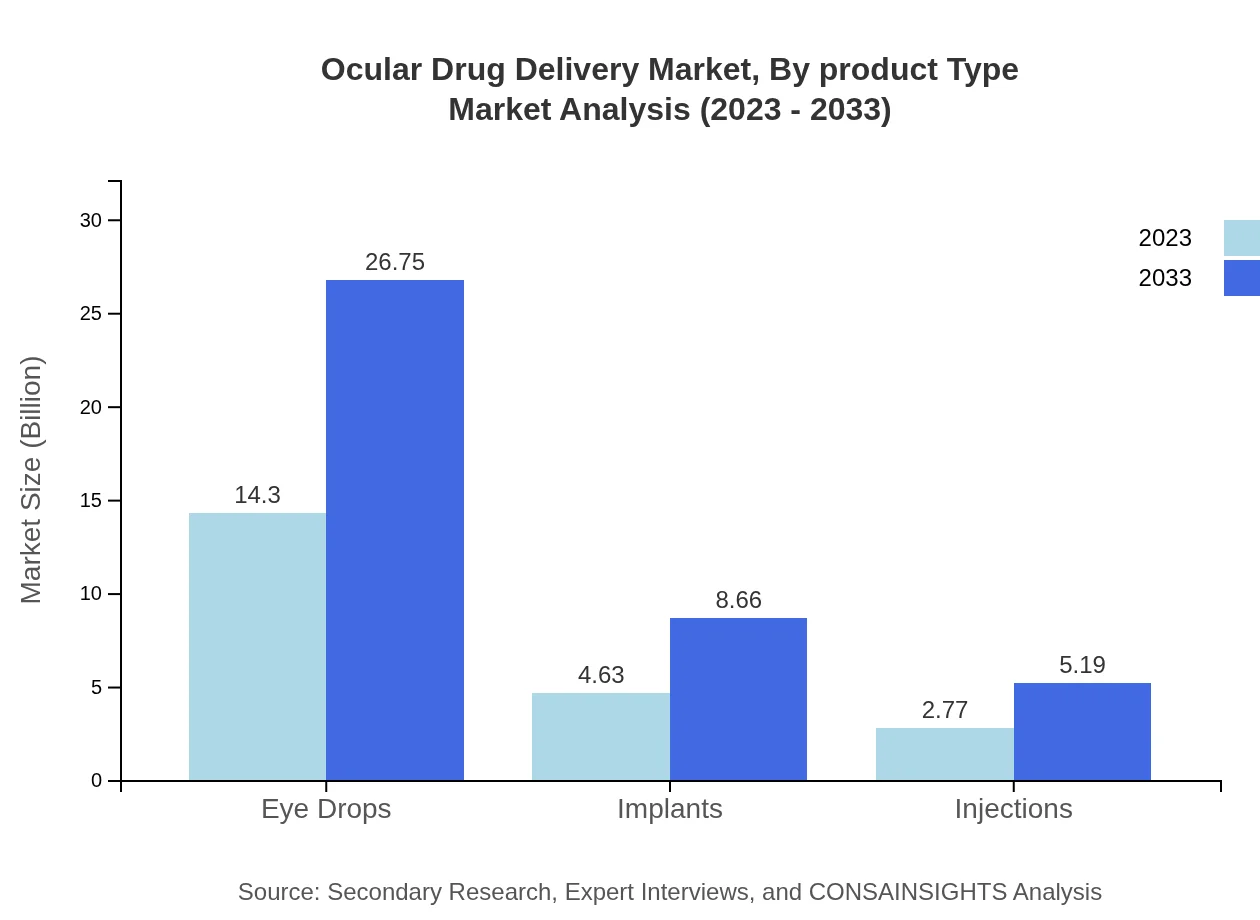
The Ocular Drug Delivery Market's product types reveal significant insights into industry dynamics. Eye drops constitute the largest segment, expected to grow from $14.30 billion in 2023 to $26.75 billion in 2033, holding a 65.88% market share. Following eye drops, implants represent a significant innovation, expected to rise from $4.63 billion to $8.66 billion, capturing a 21.34% share. Injections and topical applications also contribute to the segment's diverse landscape, underlining the evolving preferences for effective ocular drug delivery solutions.
Ocular Drug Delivery Market Analysis By Application
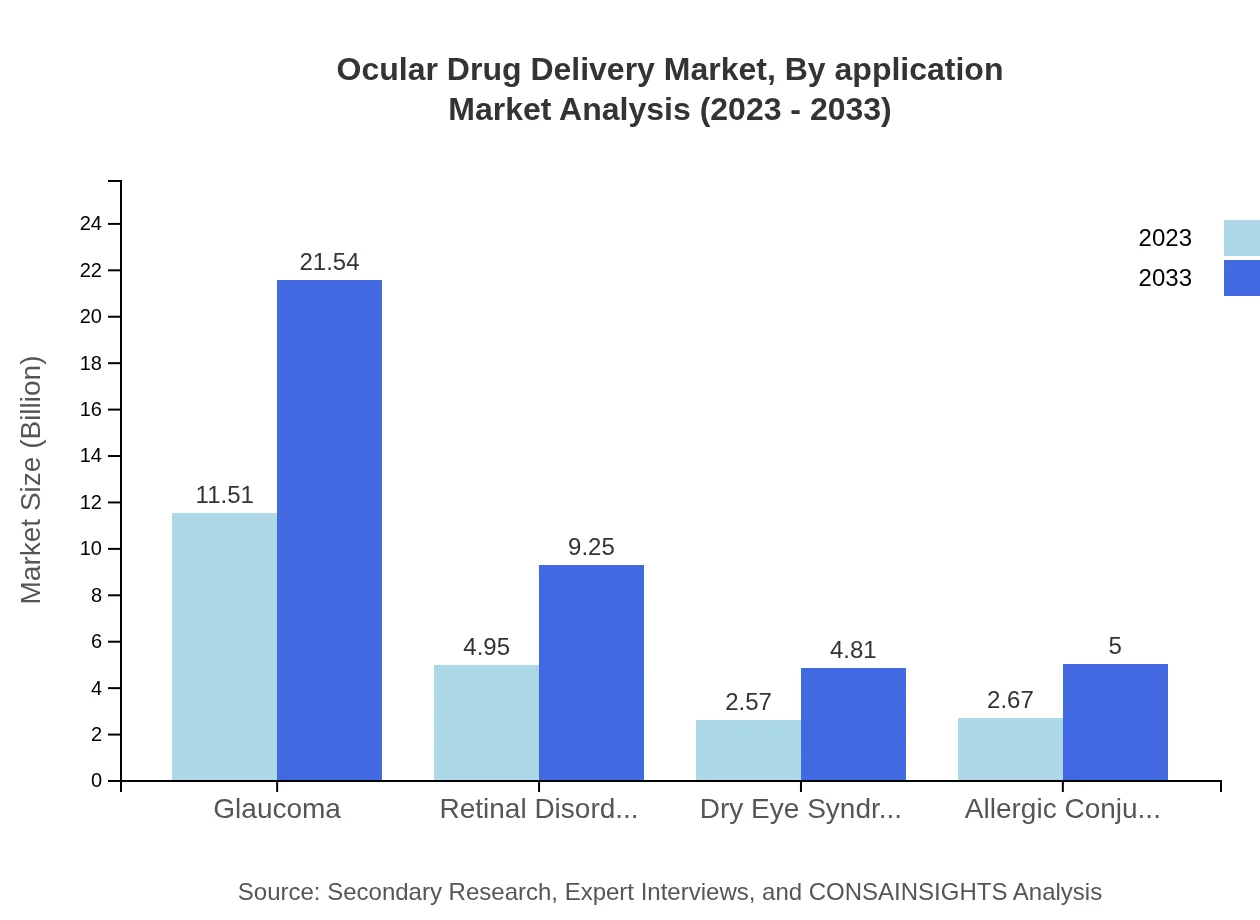
Application segmentation reflects the diverse therapeutic needs in the Ocular Drug Delivery market. Glaucoma treatment dominates the sector, expected to reach $21.54 billion by 2033 from $11.51 billion in 2023, securing a 53.05% market share overall. Retinal disorders follow, with an anticipated growth from $4.95 billion to $9.25 billion. Other applications such as dry eye syndrome and allergic conjunctivitis also contribute to segment growth, reflecting the increasing need for specialized ocular therapies.
Ocular Drug Delivery Market Analysis By Route Of Administration
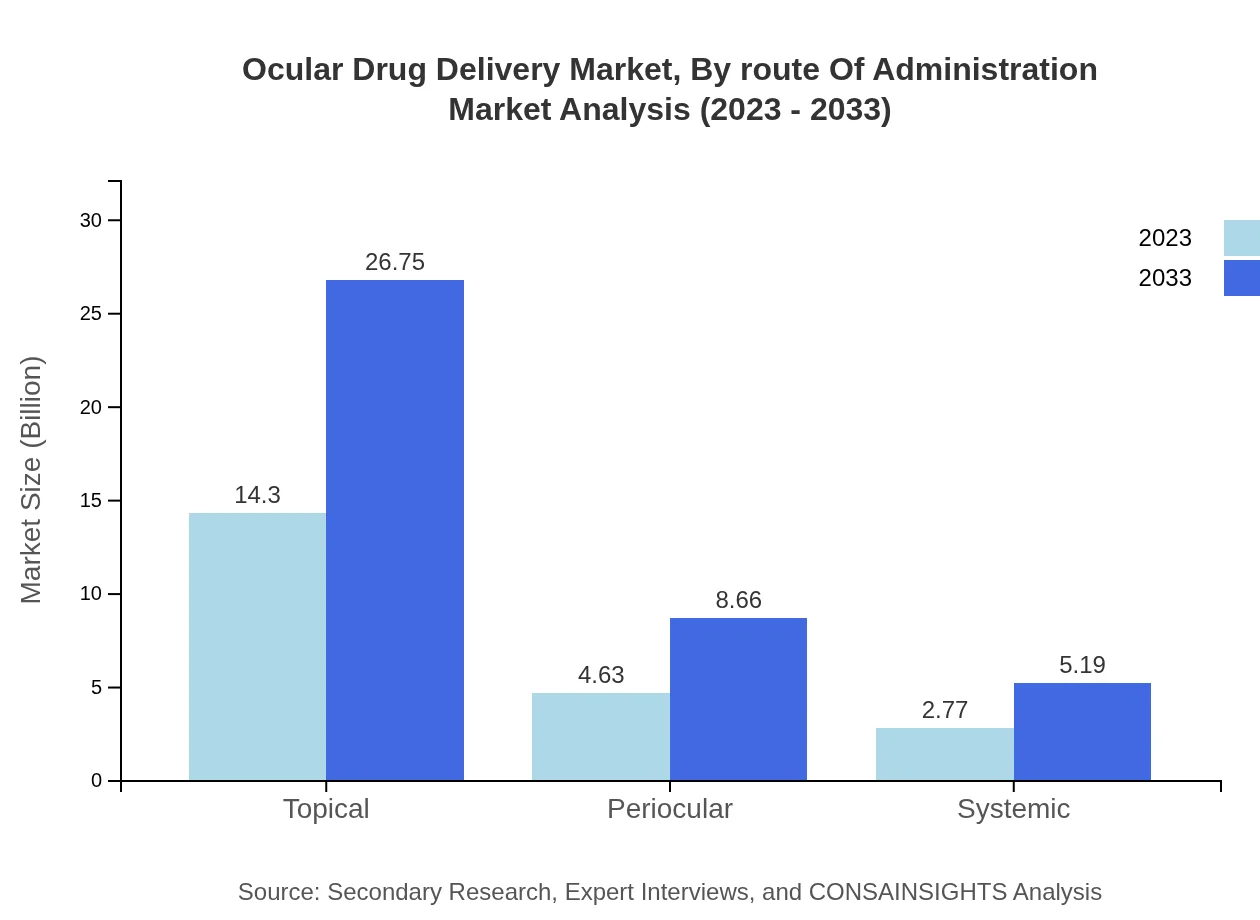
The distribution of ocular drug delivery methods showcases key trends: topical administration leads the sector with a size projection of $14.30 billion in 2023 and an expected increase to $26.75 billion by 2033. This represents a consistent share of 65.88%. In contrast, the periocular and systemic routes are gaining traction, indicating a shift in therapeutic approaches towards enhancing drug absorption and minimizing systemic effects.
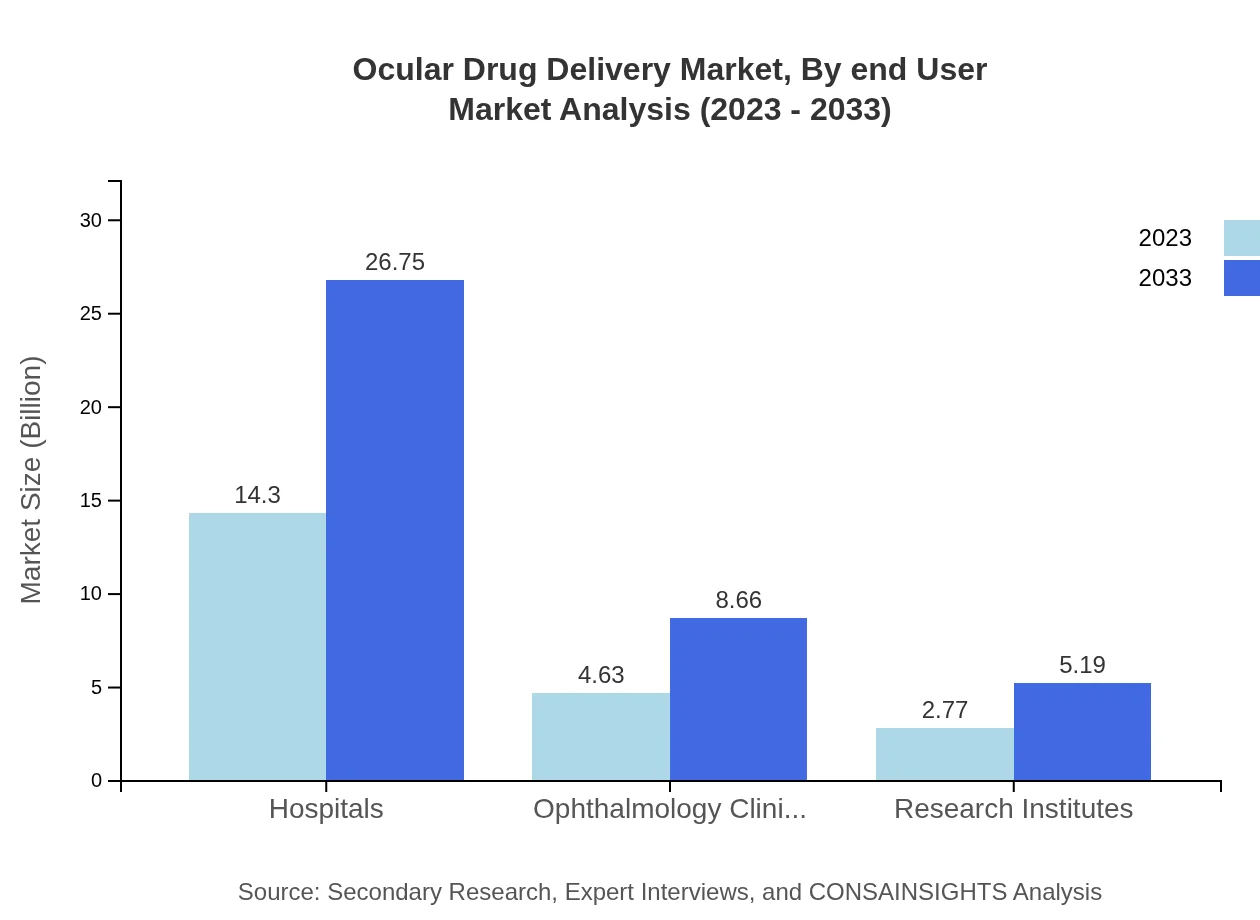
Ocular Drug Delivery Market Analysis By End User
End-user analysis identifies hospitals as the primary sectors for ocular drug delivery, with a projected growth from $14.30 billion in 2023 to $26.75 billion in 2033, maintaining a stable 65.88% share throughout the forecast period. Ophthalmology clinics are also notable contributors, increasing from $4.63 billion to $8.66 billion. Research institutes support ongoing advancements and product validation, with an expected rise from $2.77 billion to $5.19 billion.
Ocular Drug Delivery Market Analysis By Region Type
Regional segmentation emphasizes diverse growth trajectories. North America, with its advanced healthcare landscape, leads with projected revenues reaching $15.52 billion, while Europe and Asia Pacific follow with $12.26 billion and $7.02 billion, respectively. This segmentation allows stakeholders to tailor strategies and adapt to specific regional trends and demands.
Ocular Drug Delivery Market Trends and Future Forecast
Tell us your focus area and get a customized research report.
Global Market Leaders and Top Companies in Ocular Drug Delivery Industry
Allergan:
A leading pharmaceutical company known for its innovative eye care products, including treatments for glaucoma and dry eye syndrome.Novartis:
A multinational healthcare company that offers a wide range of treatment options in the ophthalmology sector, focusing on retinal diseases and surgical innovations.Bausch + Lomb:
Specializes in eye health products, including contact lenses and ocular pharmaceuticals, committed to advancing ocular health.Regeneron Pharmaceuticals:
Focused on ophthalmology, offering notable treatments for retinal diseases and conducting cutting-edge research.We're grateful to work with incredible clients.









FAQs
What is the market size of ocular drug delivery?
The ocular drug delivery market is currently valued at approximately $21.7 billion, with an expected compound annual growth rate (CAGR) of 6.3% projected over the next decade. This growth reflects increasing demand for effective ocular treatments.
What are the key market players or companies in this ocular drug delivery industry?
Key players in the ocular drug delivery market include major pharmaceutical companies specializing in ophthalmology, innovative biotechnology firms, and research institutes focusing on ocular therapies, ensuring a competitive landscape for advancements and innovations.
What are the primary factors driving the growth in the ocular drug delivery industry?
Growth in the ocular drug delivery market is driven by the rise in ocular diseases, advancements in drug formulation and delivery technologies, increasing patient populations, and trends toward non-invasive treatment methods which enhance patient compliance.
Which region is the fastest Growing in the ocular drug delivery market?
The Asia Pacific region is the fastest-growing market for ocular drug delivery, with the market projected to grow from $3.75 billion in 2023 to $7.02 billion by 2033, reflecting a growing population and advancements in healthcare.
Does ConsaInsights provide customized market report data for the ocular drug delivery industry?
Yes, ConsaInsights offers customized market reports tailored to the ocular drug delivery industry, accommodating specific client needs and providing insights that focus on individual market segments or geographic areas of interest.
What deliverables can I expect from this ocular drug delivery market research project?
Expected deliverables from the ocular drug delivery market research project include comprehensive reports detailing market size, growth forecasts, competitive analysis, segmentation data, and regional insights over the specified forecast period.
What are the market trends of ocular drug delivery?
Current trends in the ocular drug delivery market include increasing focus on patient-centric solutions, innovations in sustained-release delivery systems, and a shift towards non-invasive treatment options, thereby enhancing the effectiveness of ocular therapies.