Oncology Clinical Trials Market Report
Published Date: 31 January 2026 | Report Code: oncology-clinical-trials
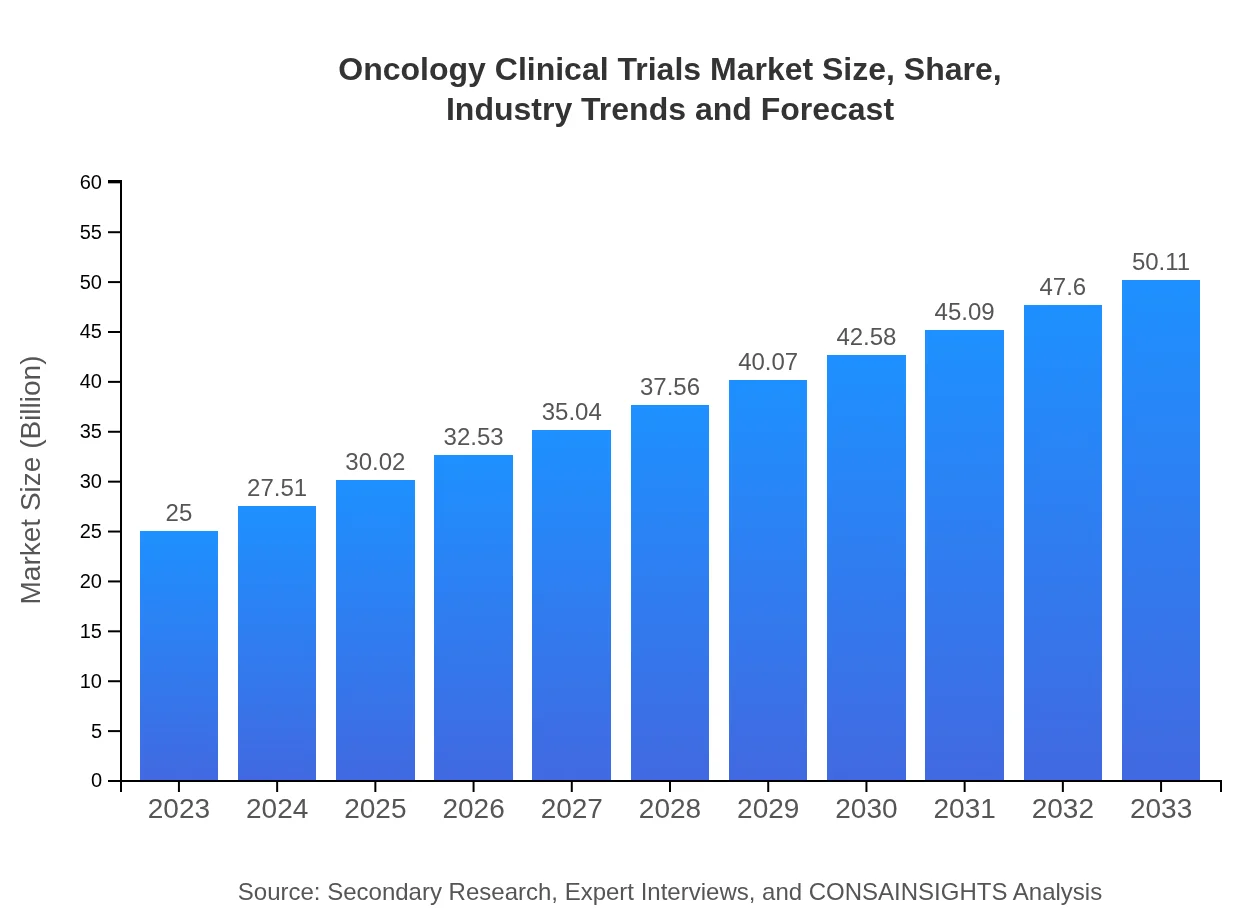
Oncology Clinical Trials Market Size, Share, Industry Trends and Forecast to 2033
This report delves into the Oncology Clinical Trials market, providing insights about its growth, segmentation, and regional performance from 2023 to 2033. It covers market size, trends, forecasts, and key players influencing the industry.
| Metric | Value |
|---|---|
| Study Period | 2023 - 2033 |
| 2023 Market Size | $25.00 Billion |
| CAGR (2023-2033) | 7% |
| 2033 Market Size | $50.11 Billion |
| Top Companies | Quintiles IMS, Covance, PAREXEL International, Medpace |
| Last Modified Date | 31 January 2026 |
Oncology Clinical Trials Market Overview
Customize Oncology Clinical Trials Market Report market research report
- ✔ Get in-depth analysis of Oncology Clinical Trials market size, growth, and forecasts.
- ✔ Understand Oncology Clinical Trials's regional dynamics and industry-specific trends.
- ✔ Identify potential applications, end-user demand, and growth segments in Oncology Clinical Trials
What is the Market Size & CAGR of Oncology Clinical Trials market in 2023?
Oncology Clinical Trials Industry Analysis
Oncology Clinical Trials Market Segmentation and Scope
Tell us your focus area and get a customized research report.
Oncology Clinical Trials Market Analysis Report by Region
Europe Oncology Clinical Trials Market Report:
Europe's oncology clinical trials market is estimated to increase from $7.49 billion in 2023 to $15.01 billion by 2033, driven by continued innovation, significant investment in research, and the presence of numerous key players. The region is also a leader in regulatory standards, enhancing trial trust and efficiency.Asia Pacific Oncology Clinical Trials Market Report:
The Asia Pacific region, valued at $4.74 billion in 2023, is expected to reach $9.51 billion by 2033. This growth is driven by the increasing prevalence of cancer in countries like China and India, alongside growing healthcare investments and demand for innovative treatments.North America Oncology Clinical Trials Market Report:
North America remains one of the largest markets, projected to grow from $9.20 billion in 2023 to $18.44 billion by 2033. The region's advanced healthcare infrastructure, robust regulatory frameworks, and substantial funding from various sources bolster its position in the oncology trials market.South America Oncology Clinical Trials Market Report:
The South American market, anticipated to grow from $1.45 billion in 2023 to $2.91 billion by 2033, is witnessing a gradual increase in clinical trial activity, encouraged by governmental initiatives and international partnerships aimed at improving healthcare standards.Middle East & Africa Oncology Clinical Trials Market Report:
The market in the Middle East and Africa, starting at $2.12 billion in 2023, is projected to double to $4.24 billion by 2033. The initiatives aimed at boosting cancer research, along with international collaborations, are crucial for the region's growth.Tell us your focus area and get a customized research report.
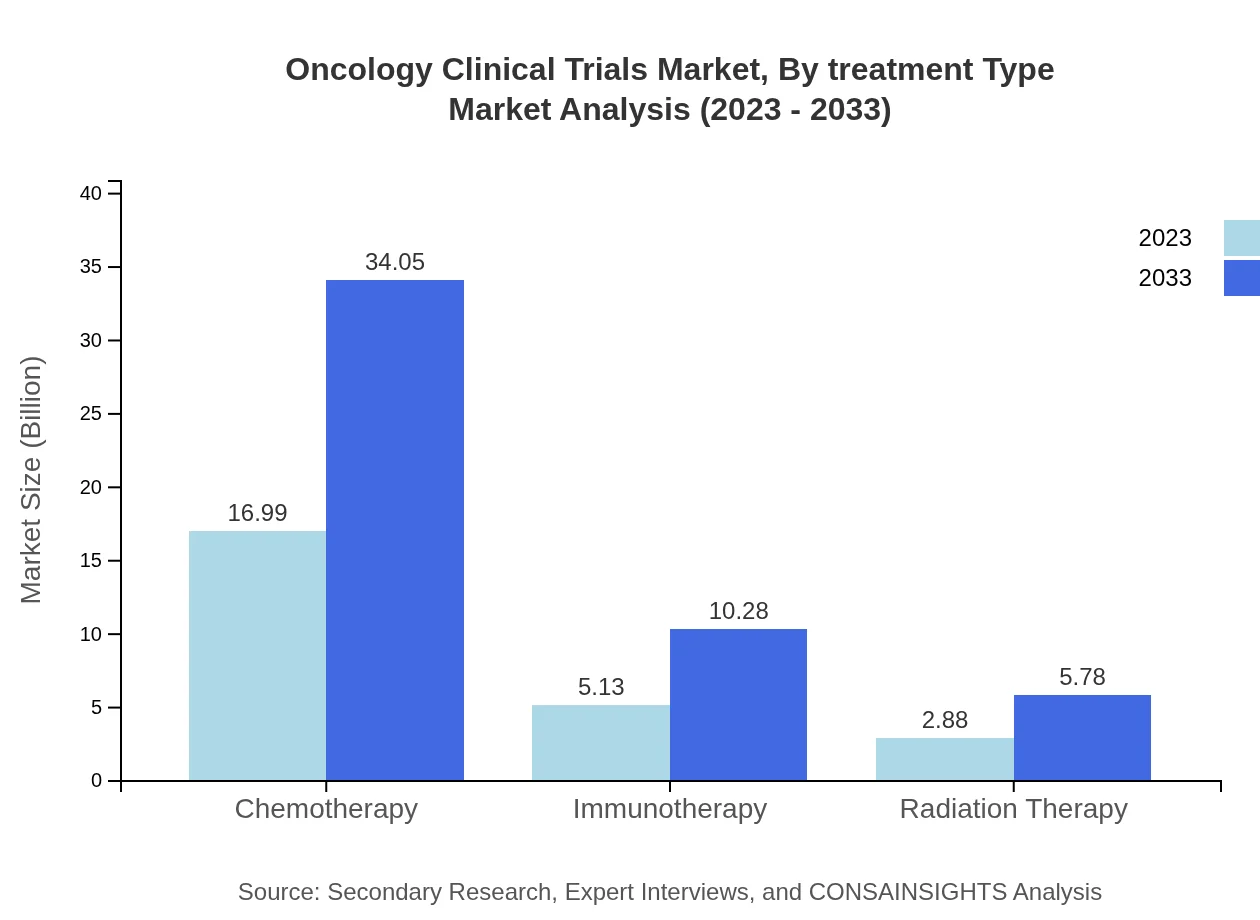
Oncology Clinical Trials Market Analysis By Treatment Type
The treatment type segment reveals that chemotherapy dominates the Oncology Clinical Trials market, with a market size of $16.99 billion in 2023, expected to increase to $34.05 billion by 2033. Immunotherapy and radiation therapy are also significant, indicating a strong inclination towards targeted treatments in clinical research.
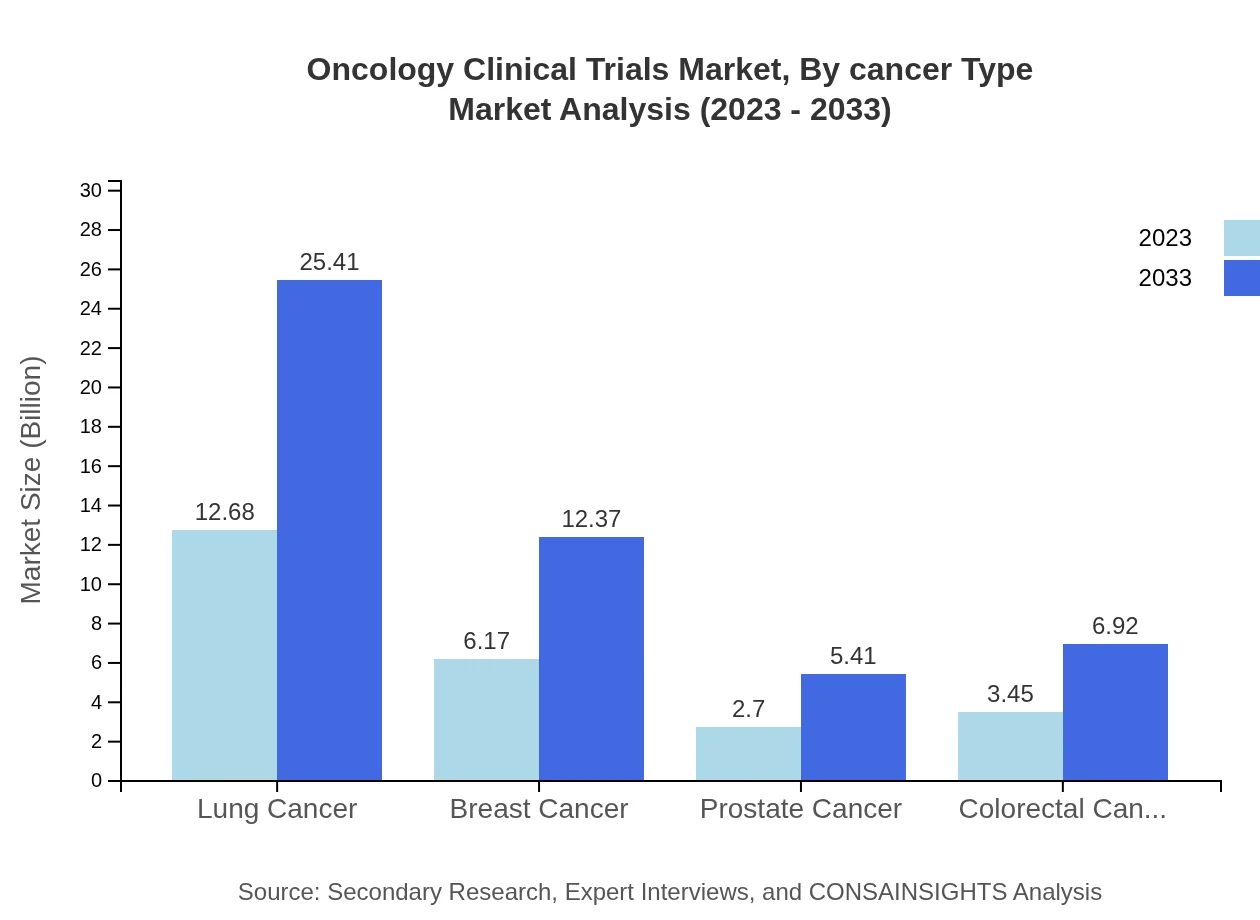
Oncology Clinical Trials Market Analysis By Cancer Type
The cancer type segment shows lung cancer as the largest contributor, with a market size of $12.68 billion in 2023, scaling up to $25.41 billion by 2033. Other notable types include breast cancer, highlighting a collective shift towards addressing high-prevalence malignancies in clinical trials.
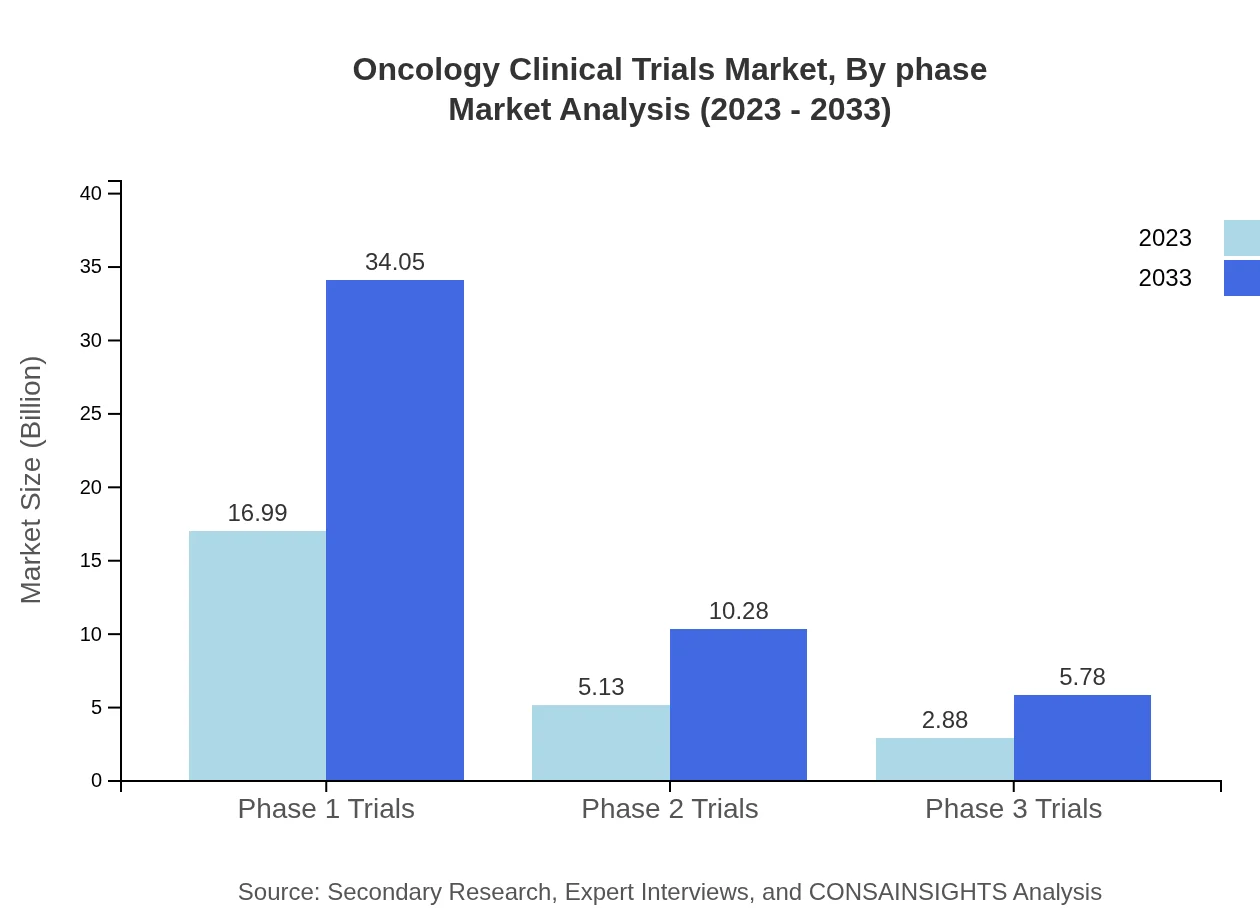
Oncology Clinical Trials Market Analysis By Phase
Focusing on trial phases, Phase 1 trials represent a dominant segment, starting at $16.99 billion in 2023 and expected to reach $34.05 billion by 2033. This phase contributes significantly due to an increasing number of compounds entering trials aimed at assessing safety and determining dose.
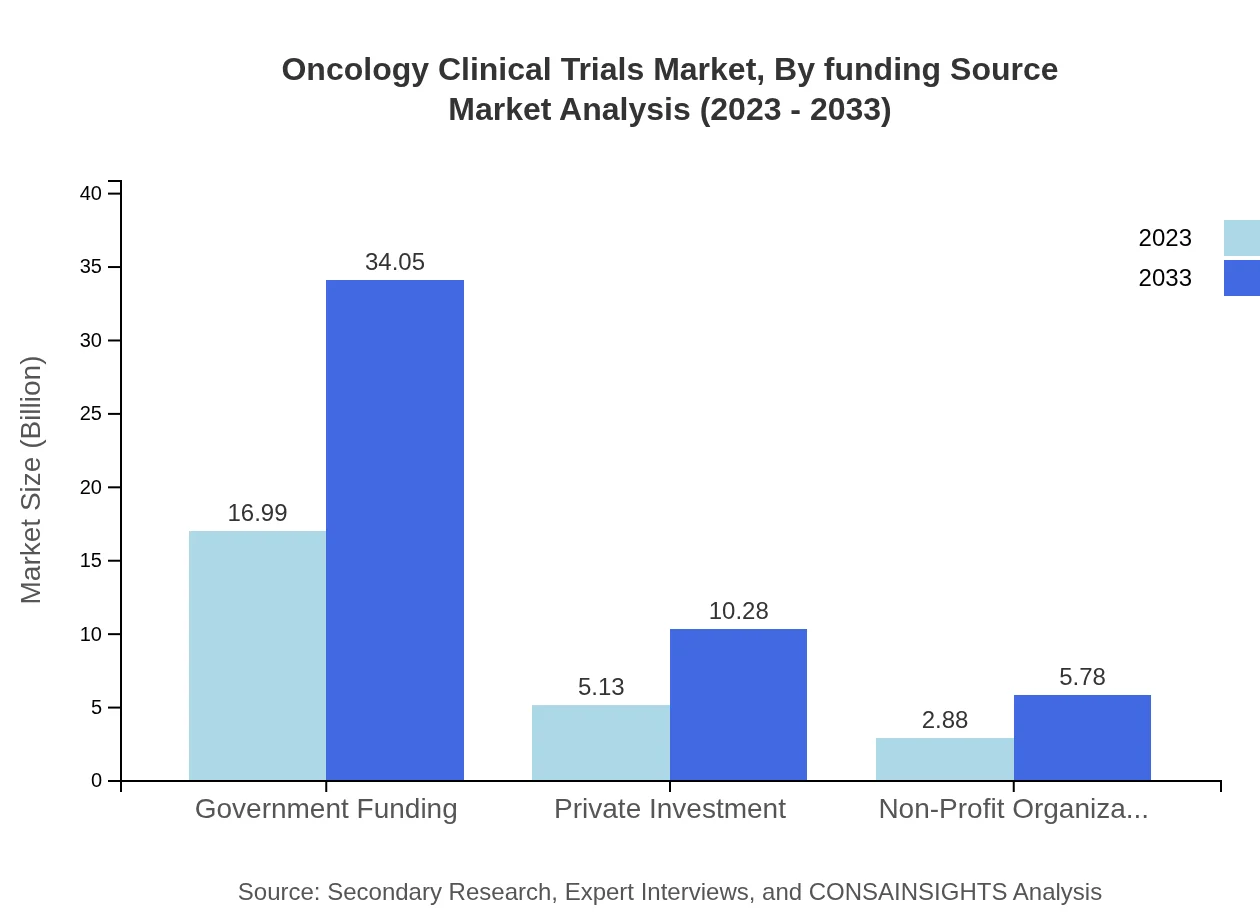
Oncology Clinical Trials Market Analysis By Funding Source
The funding source segment highlights that government funding leads the industry, with a market share of $16.99 billion in 2023 anticipated to grow to $34.05 billion by 2033. Private investment and funds from non-profit organizations are also notable, demonstrating collaborative efforts to address cancer research funding.
Oncology Clinical Trials Market Trends and Future Forecast
Tell us your focus area and get a customized research report.
Global Market Leaders and Top Companies in Oncology Clinical Trials Industry
Quintiles IMS:
One of the world's largest contract research organizations (CRO), providing comprehensive services for clinical trials, including patient recruitment and data management.Covance:
A global leader in drug development services, Covance offers a wide range of solutions, from preclinical to clinical research, to accelerate new cancer therapy development.PAREXEL International:
PAREXEL specializes in providing consulting, strategy, and clinical research services focused on the pharmaceutical and biotechnology sectors, aiding in the efficient execution of oncology trials.Medpace:
An innovative, full-service clinical research organization that provides comprehensive drug development services to the pharmaceutical and biotechnology sectors focused on oncology.We're grateful to work with incredible clients.









FAQs
What is the market size of oncology clinical trials?
The oncology clinical trials market size is projected to grow from $25 billion in 2023 to significant levels by 2033, with a robust CAGR of 7%. This growth underscores the increasing focus on developing innovative cancer therapies.
What are the key market players or companies in the oncology clinical trials industry?
Key players in the oncology clinical trials sector include major pharmaceutical corporations, biotechnology firms, and specialized contract research organizations (CROs). These companies drive innovation and research in cancer therapies, collaborating globally to enhance trial outcomes.
What are the primary factors driving the growth in the oncology clinical trials industry?
Growth in the oncology clinical trials industry is primarily driven by increasing cancer prevalence, advances in technology, regulatory support for drug approvals, and rising investment from both governmental entities and private sectors.
Which region is the fastest Growing in oncology clinical trials?
The fastest-growing region in the oncology clinical trials market is projected to be North America, expanding from $9.20 billion in 2023 to approximately $18.44 billion by 2033, reflecting substantial investments and research initiatives in cancer treatment.
Does ConsaInsights provide customized market report data for the oncology clinical trials industry?
Yes, ConsaInsights offers customized market report data tailored to the oncology clinical trials industry. Clients can request specific insights, trends, and analytics suited to their business needs, ensuring they receive the most relevant information.
What deliverables can I expect from this oncology clinical trials market research project?
Expect deliverables such as detailed market analysis, segmentation data, trend reports, regional insights, and strategic recommendations. These insights can help guide investment decisions and strategic planning in the oncology clinical trials market.
What are the market trends of oncology clinical trials?
Current market trends in oncology clinical trials include a surge in adaptive trial designs, increased use of real-world evidence, and the integration of artificial intelligence in clinical research processes, reflecting the industry's evolution towards more efficient and patient-centric approaches.