Oral Thin Film Drugs Market Report
Published Date: 31 January 2026 | Report Code: oral-thin-film-drugs
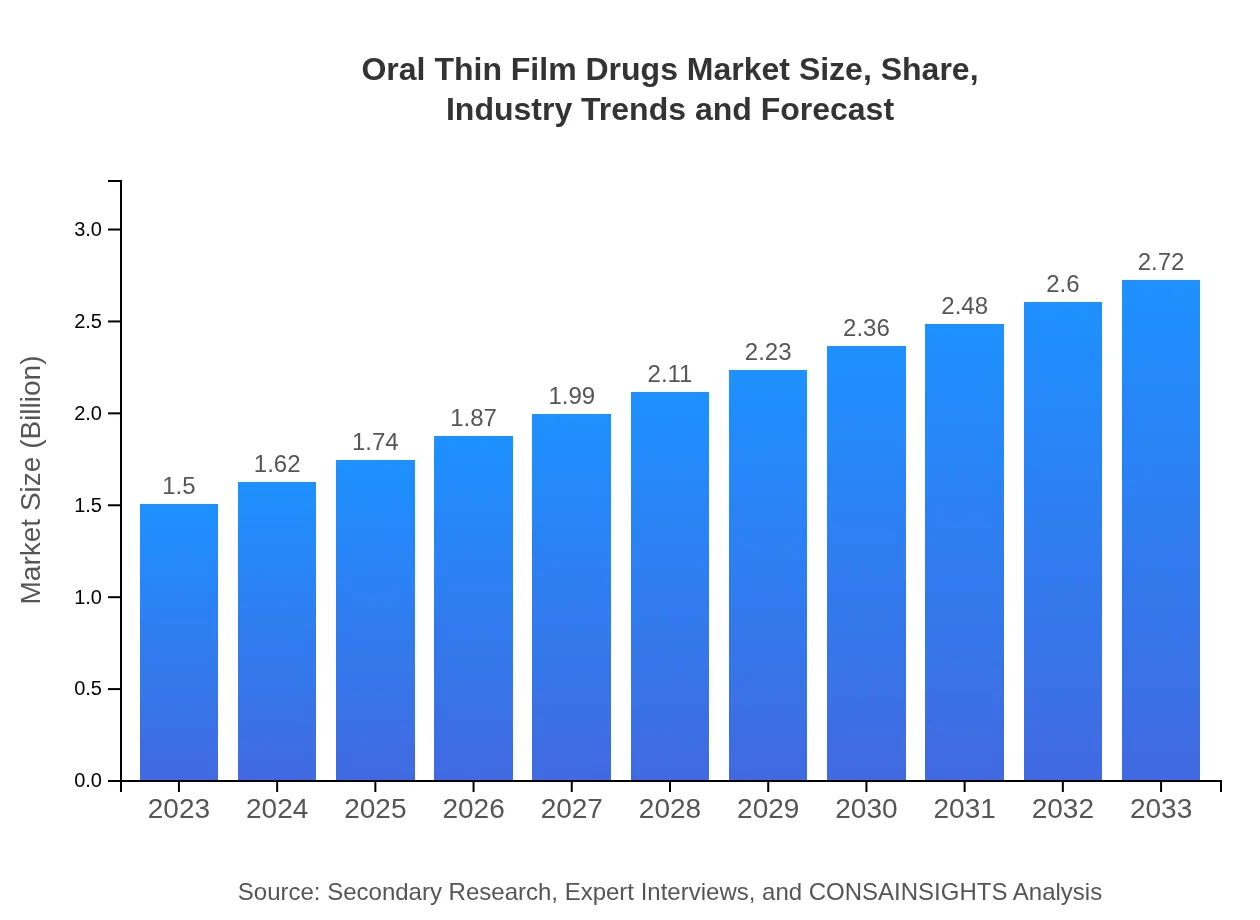
Oral Thin Film Drugs Market Size, Share, Industry Trends and Forecast to 2033
This report provides a comprehensive analysis of the Oral Thin Film Drugs market from 2023 to 2033, exploring market size, growth trends, segmentation, and regional insights, alongside technology and product analysis, competitive landscape, and future forecasts.
| Metric | Value |
|---|---|
| Study Period | 2023 - 2033 |
| 2023 Market Size | $1.50 Billion |
| CAGR (2023-2033) | 6.0% |
| 2033 Market Size | $2.72 Billion |
| Top Companies | Pfizer Inc., ProStrakan Group |
| Last Modified Date | 31 January 2026 |
Oral Thin Film Drugs Market Overview
Customize Oral Thin Film Drugs Market Report market research report
- ✔ Get in-depth analysis of Oral Thin Film Drugs market size, growth, and forecasts.
- ✔ Understand Oral Thin Film Drugs's regional dynamics and industry-specific trends.
- ✔ Identify potential applications, end-user demand, and growth segments in Oral Thin Film Drugs
What is the Market Size & CAGR of Oral Thin Film Drugs Market in 2023?
Oral Thin Film Drugs Industry Analysis
Oral Thin Film Drugs Market Segmentation and Scope
Tell us your focus area and get a customized research report.
Oral Thin Film Drugs Market Analysis Report by Region
Europe Oral Thin Film Drugs Market Report:
Europe's market stood at $0.51 billion in 2023 and is anticipated to reach $0.92 billion by 2033. The region's growth is attributed to the increasing acceptance of oral thin films in therapeutic applications and supportive regulatory frameworks.Asia Pacific Oral Thin Film Drugs Market Report:
In the Asia Pacific region, the market valuation stood at $0.28 billion in 2023 and is projected to reach $0.51 billion by 2033, reflecting a growing interest in advanced drug delivery systems driven by increasing healthcare investments and awareness.North America Oral Thin Film Drugs Market Report:
North America is a significant market with a size of $0.52 billion in 2023, projected to grow to $0.94 billion by 2033. The region's growth is primarily supported by robust healthcare infrastructures and a drive towards innovative drug delivery solutions.South America Oral Thin Film Drugs Market Report:
The South American market was valued at $0.08 billion in 2023 and is expected to grow to $0.15 billion by 2033, bolstered by the rising prevalence of chronic diseases and demand for effective treatment options.Middle East & Africa Oral Thin Film Drugs Market Report:
In the Middle East and Africa, the market was valued at $0.12 billion in 2023, expected to reach $0.21 billion by 2033. The healthcare sector is evolving, leading to a rising demand for effective and patient-friendly medication solutions in the region.Tell us your focus area and get a customized research report.
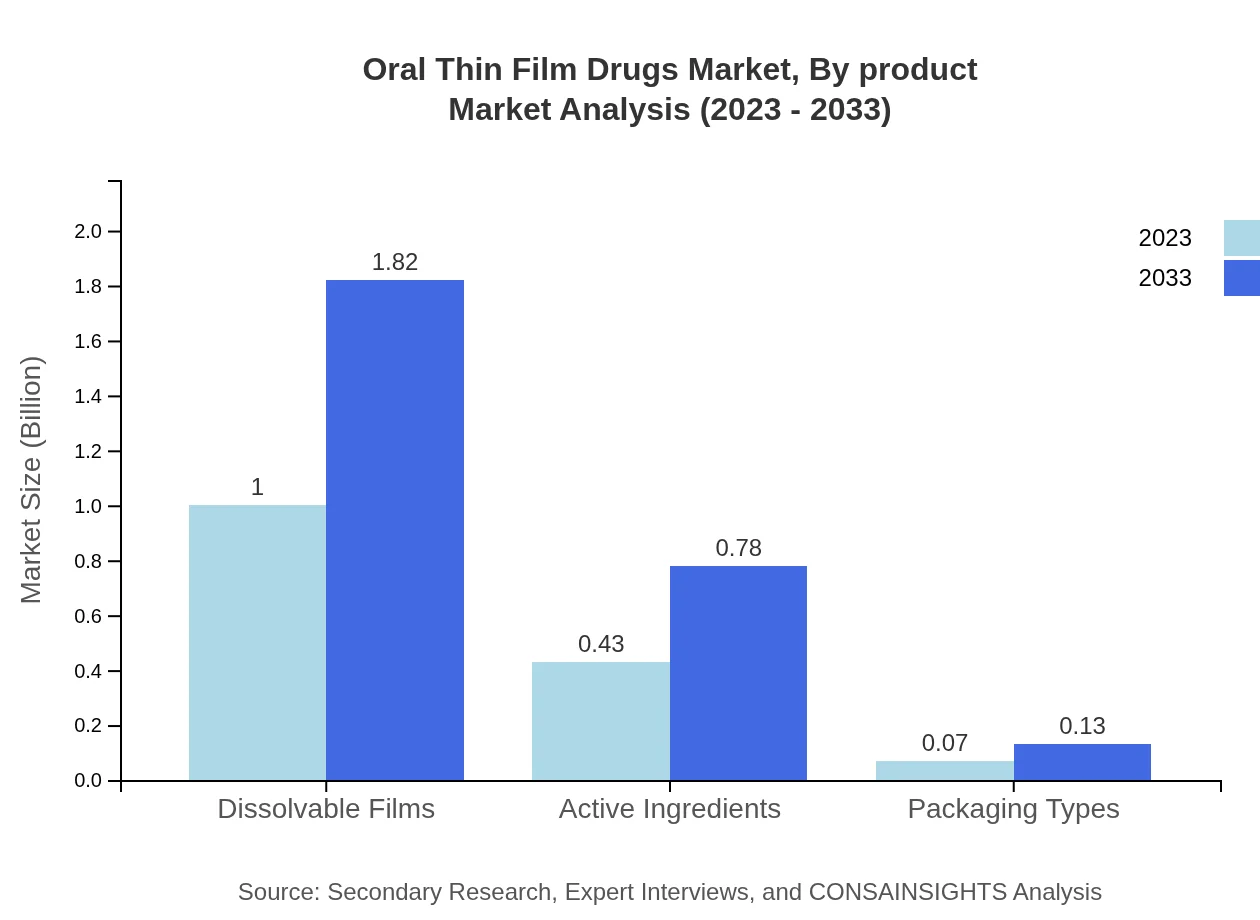
Oral Thin Film Drugs Market Analysis By Product
The segment for dissolvable films dominates the Oral Thin Film Drugs market, accounting for $1.00 billion in 2023 and projected to reach $1.82 billion by 2033. Dissolvable films hold a 66.62% market share, indicating their significant impact in facilitating rapid drug absorption and improving patient adherence to treatment regimens.
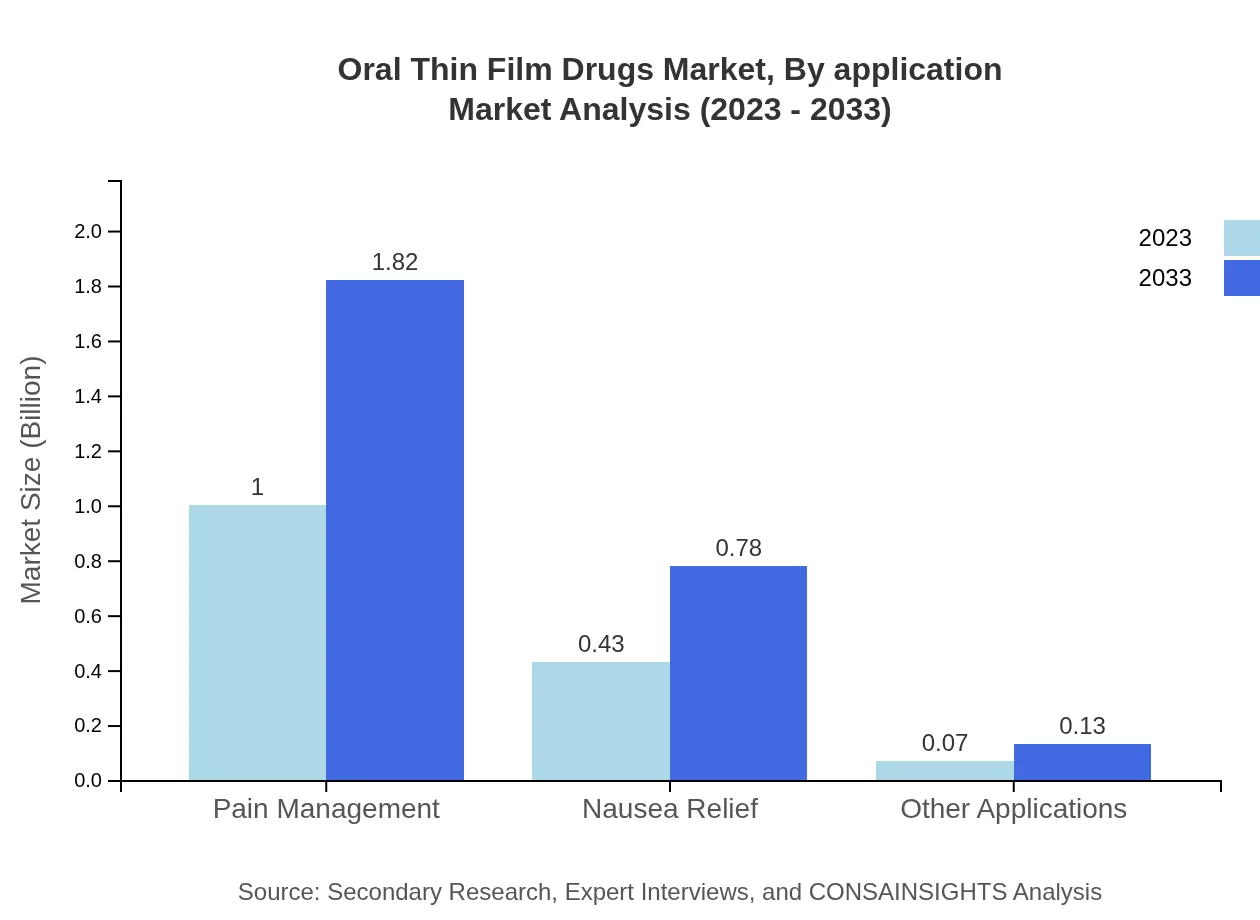
Oral Thin Film Drugs Market Analysis By Application
Application-wise, pain management dominates with a share of 66.62% in 2023, expected to maintain the same share through 2033. This reflects the critical use of oral thin films in managing conditions requiring rapid analgesic delivery. Other notable applications include nausea relief, accounting for 28.72%, and other therapeutic areas reflecting growing patient needs.
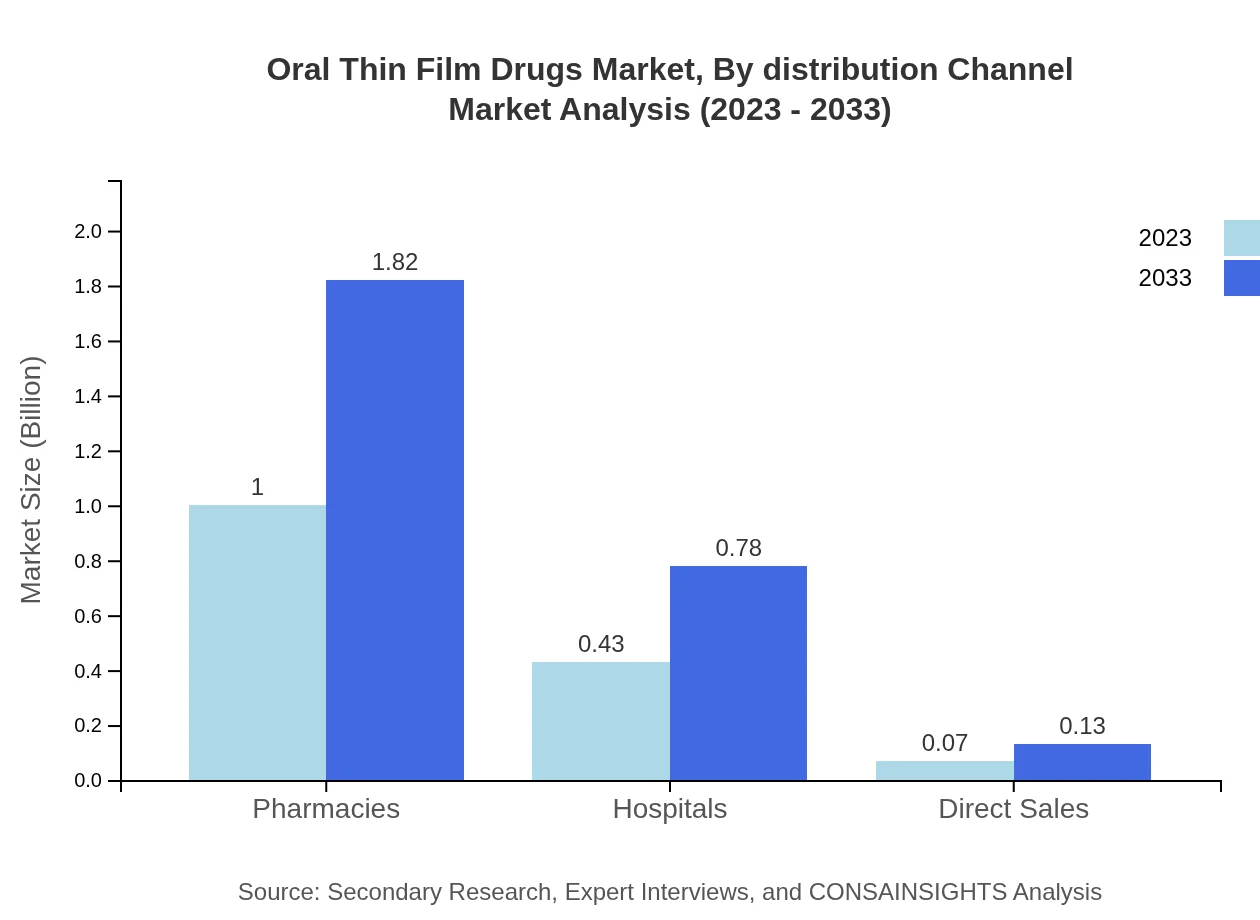
Oral Thin Film Drugs Market Analysis By Distribution Channel
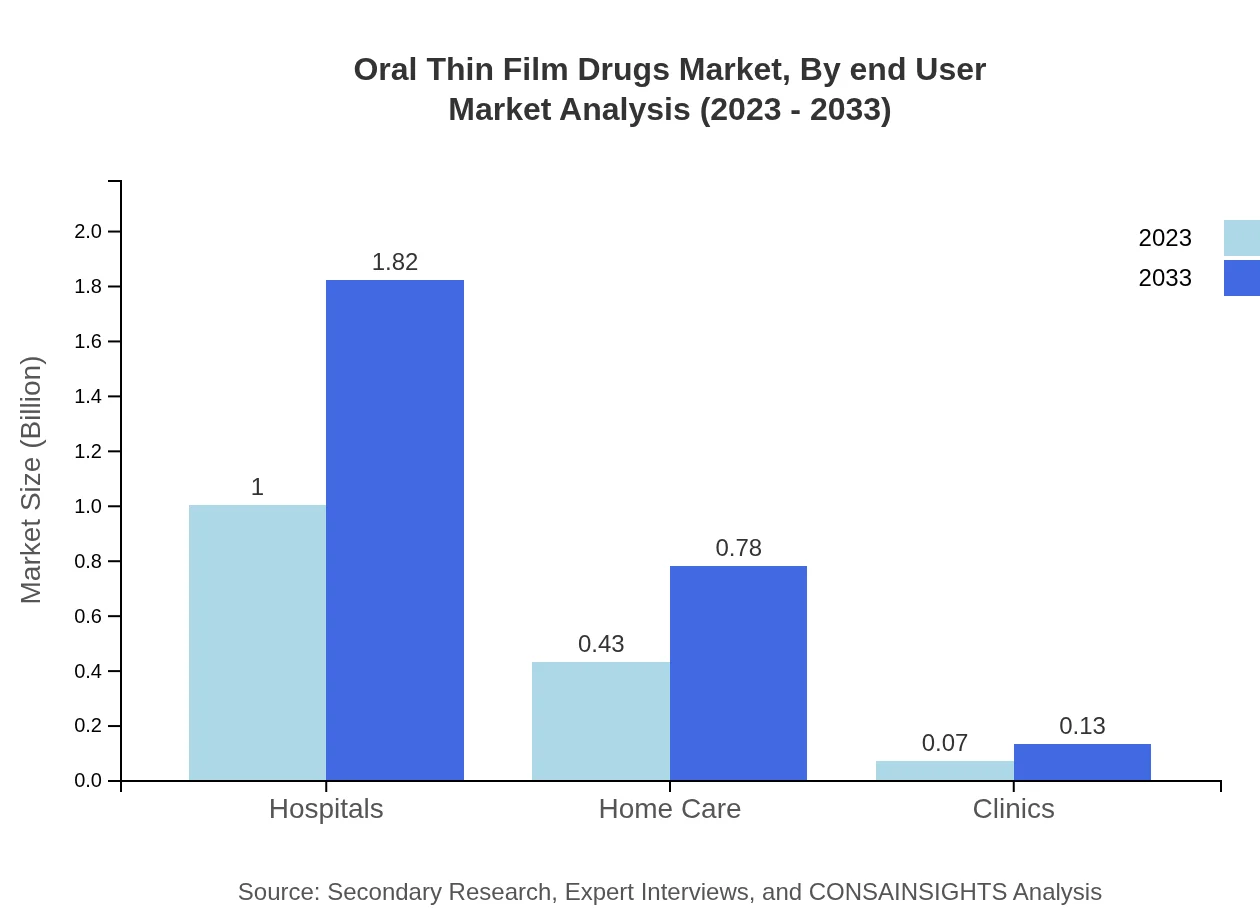
Hospitals represent a key distribution channel, holding $1.00 billion in size for 2023, projected to grow to $1.82 billion by 2033, indicating their role in administering effective drug delivery solutions directly to patients.
Oral Thin Film Drugs Market Analysis By End User
Pharmacies are a crucial end-user segment, with sizes expected to multiply from $1.00 billion in 2023 to $1.82 billion by 2033, marking a significant share of 66.62%, while hospitals also contribute substantially to the market.
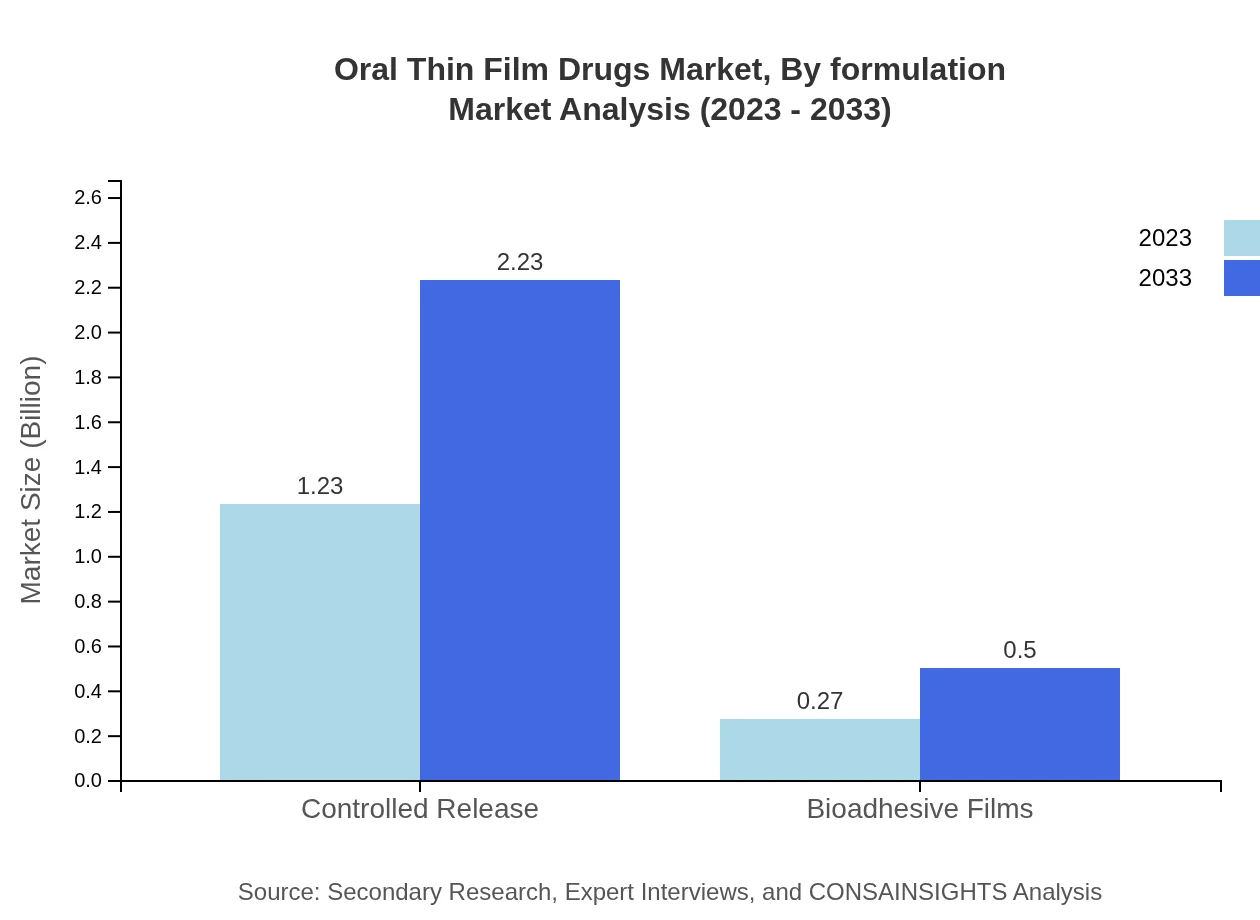
Oral Thin Film Drugs Market Analysis By Formulation
Controlled release formulations dominate the oral thin film market with an impressive revenue projection from $1.23 billion in 2023 to $2.23 billion by 2033, serving a vital role in sustaining drug release over extended periods, beneficial in various therapeutic contexts.
Oral Thin Film Drugs Market Trends and Future Forecast
Tell us your focus area and get a customized research report.
Global Market Leaders and Top Companies in Oral Thin Film Drugs Industry
Pfizer Inc.:
A global leader in pharmaceuticals, Pfizer engages extensively in developing oral thin film medications, leveraging its vast research resources to enhance drug delivery effectiveness.ProStrakan Group:
ProStrakan specializes in innovative formulations and is a prominent player in the oral thin film sector, focusing on drugs that effectively meet patient needs for chronic pain management.We're grateful to work with incredible clients.









FAQs
What is the market size of oral Thin Film Drugs?
The market size of oral thin film drugs is currently valued at $1.5 billion, with a projected CAGR of 6.0% from 2023 to 2033, indicating robust growth driven by innovation and increasing demand.
What are the key market players or companies in this oral Thin Film Drugs industry?
Key players in the oral thin film drugs industry include major pharmaceutical companies focused on innovative drug delivery mechanisms, with investments in R&D and collaborations aiming to capture growing market segments.
What are the primary factors driving the growth in the oral Thin Film Drugs industry?
Factors driving growth in the oral thin film drugs market include increased patient compliance, technological advancements in drug formulation, and rising demand for convenient dosage forms among patients.
Which region is the fastest Growing in the oral Thin Film Drugs?
The Asia Pacific region is the fastest-growing market for oral thin film drugs, with expected growth from $0.28 billion in 2023 to $0.51 billion by 2033, reflecting significant market potential.
Does Consainsights provide customized market report data for the oral Thin Film Drugs industry?
Yes, Consainsights offers customized market reports for the oral thin film drugs industry that cater to specific client needs and provide tailored insights based on individual business requirements.
What deliverables can I expect from this oral Thin Film Drugs market research project?
Deliberables from the market research project include detailed market analysis reports, trend identification, forecasts, and strategic recommendations tailored for the oral thin film drugs sector.
What are the market trends of oral Thin Film Drugs?
Current market trends in oral thin film drugs include advancements in bioadhesive films, the rise of controlled release formulations, and significant growth in demand across hospitals and pharmacies.