Orphan Drugs Market Report
Published Date: 31 January 2026 | Report Code: orphan-drugs
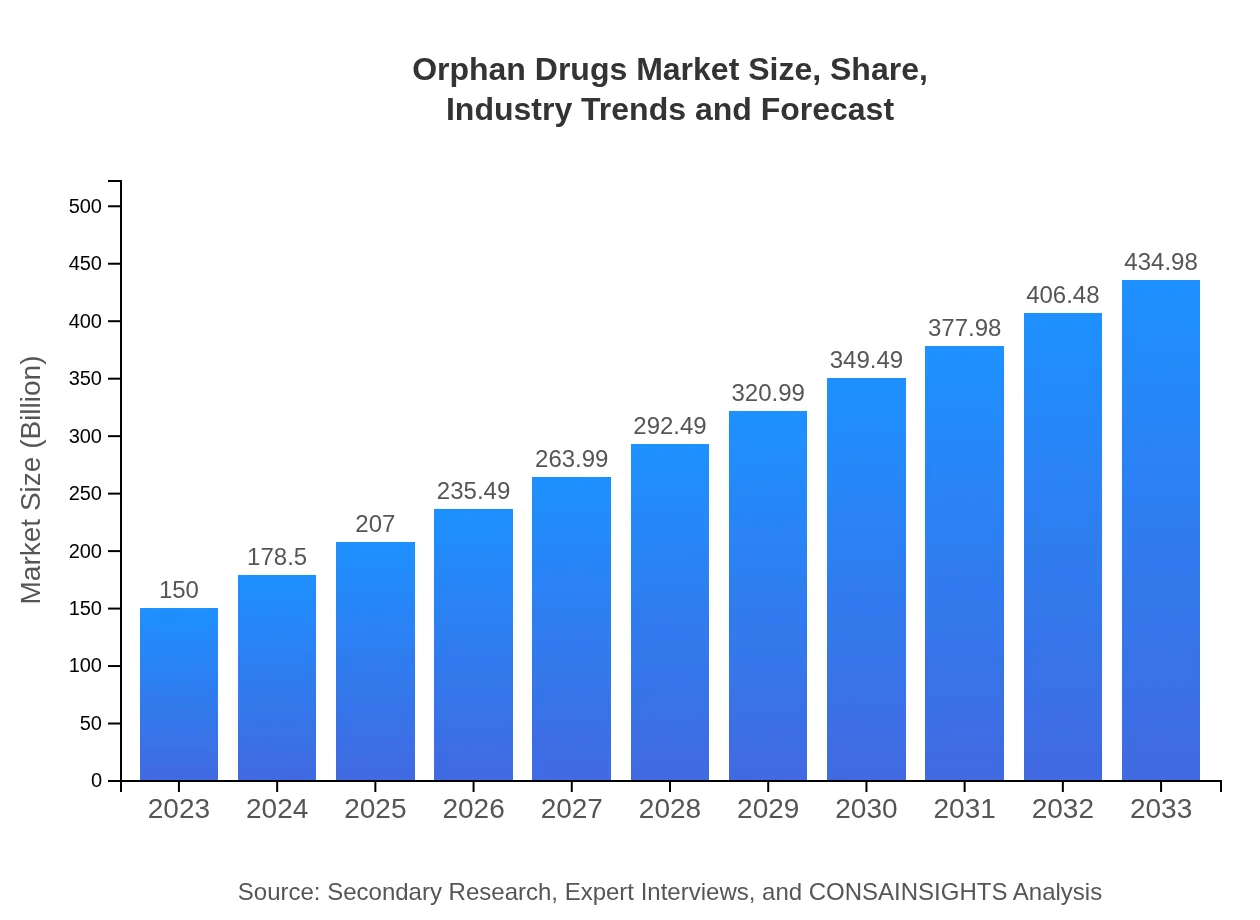
Orphan Drugs Market Size, Share, Industry Trends and Forecast to 2033
This report provides a comprehensive analysis of the orphan drugs market, focusing on market size, growth trends, segmentation, and forecasts from 2023 to 2033. Insights include regional analysis and profiles of key players within the industry.
| Metric | Value |
|---|---|
| Study Period | 2023 - 2033 |
| 2023 Market Size | $150.00 Billion |
| CAGR (2023-2033) | 10.8% |
| 2033 Market Size | $434.98 Billion |
| Top Companies | Novartis, Roche, Sanofi, Amgen, Bristol-Myers Squibb |
| Last Modified Date | 31 January 2026 |
Orphan Drugs Market Overview
Customize Orphan Drugs Market Report market research report
- ✔ Get in-depth analysis of Orphan Drugs market size, growth, and forecasts.
- ✔ Understand Orphan Drugs's regional dynamics and industry-specific trends.
- ✔ Identify potential applications, end-user demand, and growth segments in Orphan Drugs
What is the Market Size & CAGR of Orphan Drugs market in 2023?
Orphan Drugs Industry Analysis
Orphan Drugs Market Segmentation and Scope
Tell us your focus area and get a customized research report.
Orphan Drugs Market Analysis Report by Region
Europe Orphan Drugs Market Report:
The European orphan drugs market is significant, valued at $42.81 billion in 2023, growing to $124.14 billion by 2033. The region benefits from favorable regulations and a proactive approach to rare disease management.Asia Pacific Orphan Drugs Market Report:
In the Asia Pacific region, the orphan drugs market was valued at $30.66 billion in 2023 and is projected to reach $88.91 billion by 2033, driven by increasing investments in healthcare infrastructure and rising incidences of rare diseases, especially in developing countries.North America Orphan Drugs Market Report:
North America holds the largest market share, valued at $53.70 billion in 2023 and anticipated to reach $155.72 billion by 2033. This growth is primarily driven by robust research activity, strong compensation frameworks, and a mature healthcare system.South America Orphan Drugs Market Report:
The South American orphan drugs market is currently valued at $9.81 billion and is expected to grow to $28.45 billion by 2033. Growth is fueled by government initiatives to enhance rare disease awareness and improve access to treatments.Middle East & Africa Orphan Drugs Market Report:
The Middle East and Africa orphan drugs market is valued at $13.02 billion in 2023 and is expected to reach $37.76 billion by 2033. Enhancements in healthcare access and increasing investments in rare diseases are primary contributors to growth.Tell us your focus area and get a customized research report.
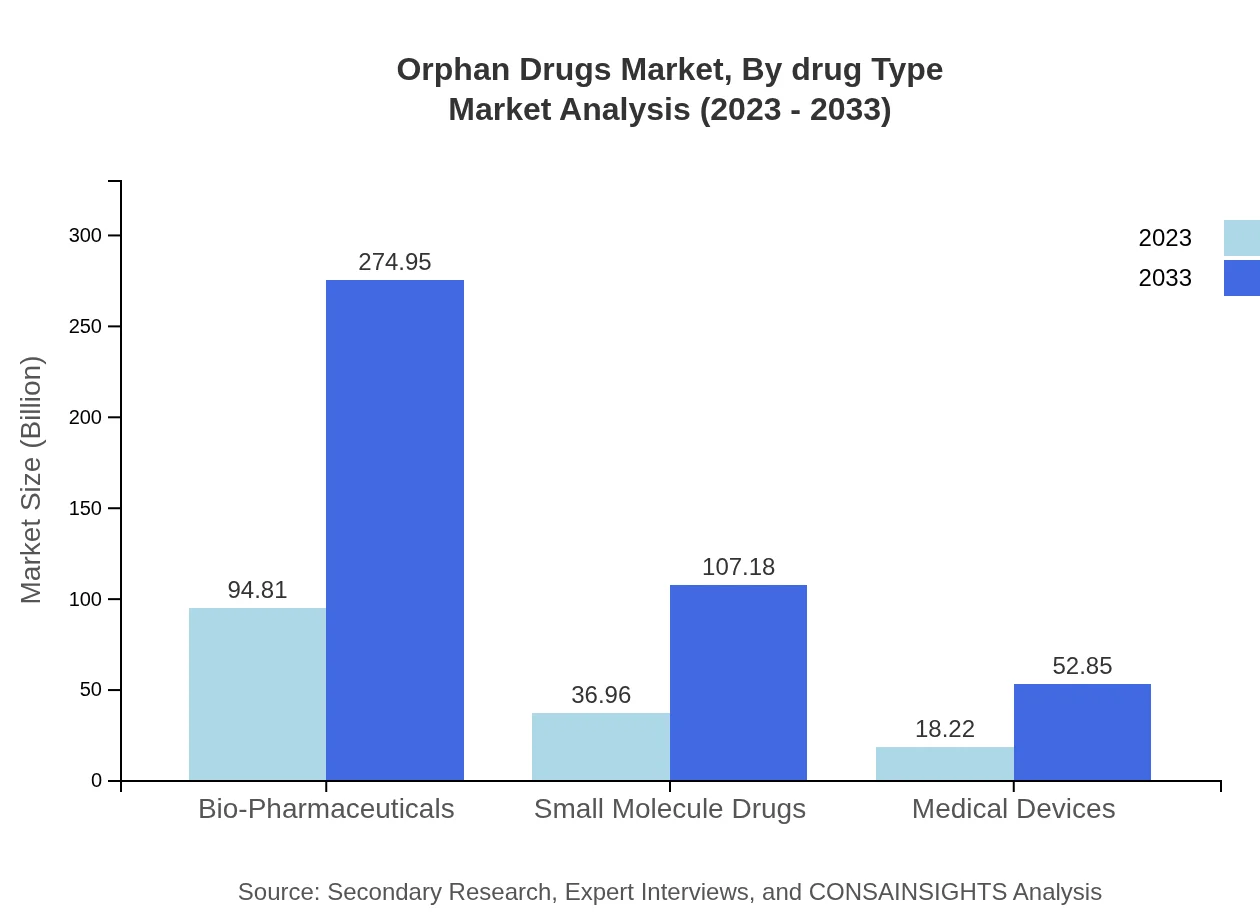
Orphan Drugs Market Analysis By Drug Type
By drug type, the orphan drugs market is dominated by biologics, which accounted for a major share of the market in 2023 due to their effectiveness in treating complex diseases. Small molecule drugs are also significant contributors, showcasing advancements in drug development technologies.
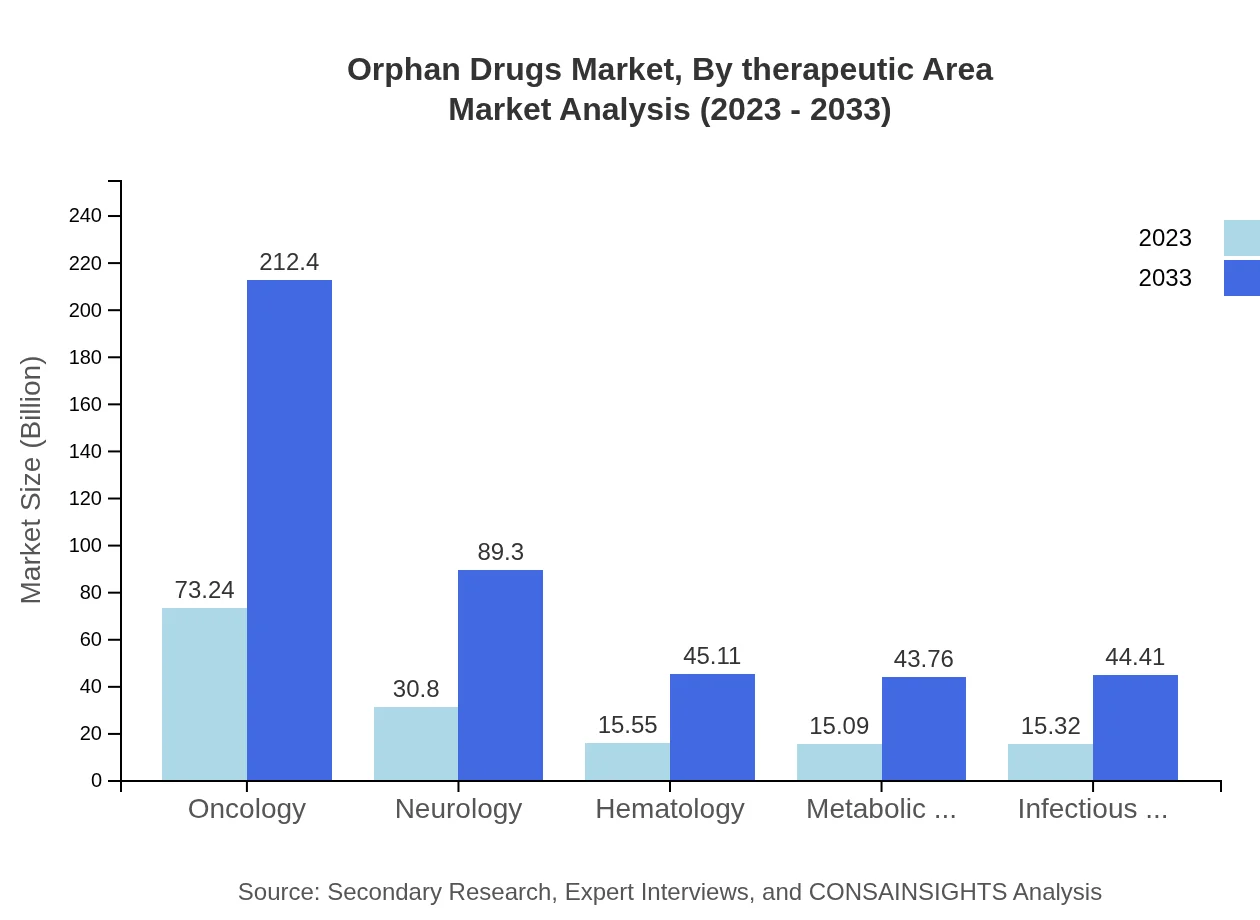
Orphan Drugs Market Analysis By Therapeutic Area
The oncology segment leads the orphan drugs market, valued at $73.24 billion in 2023 and projected to rise significantly through 2033. Neurology and hematology also contribute relevant shares, reflecting the urgent need for treatments in these therapeutic areas.
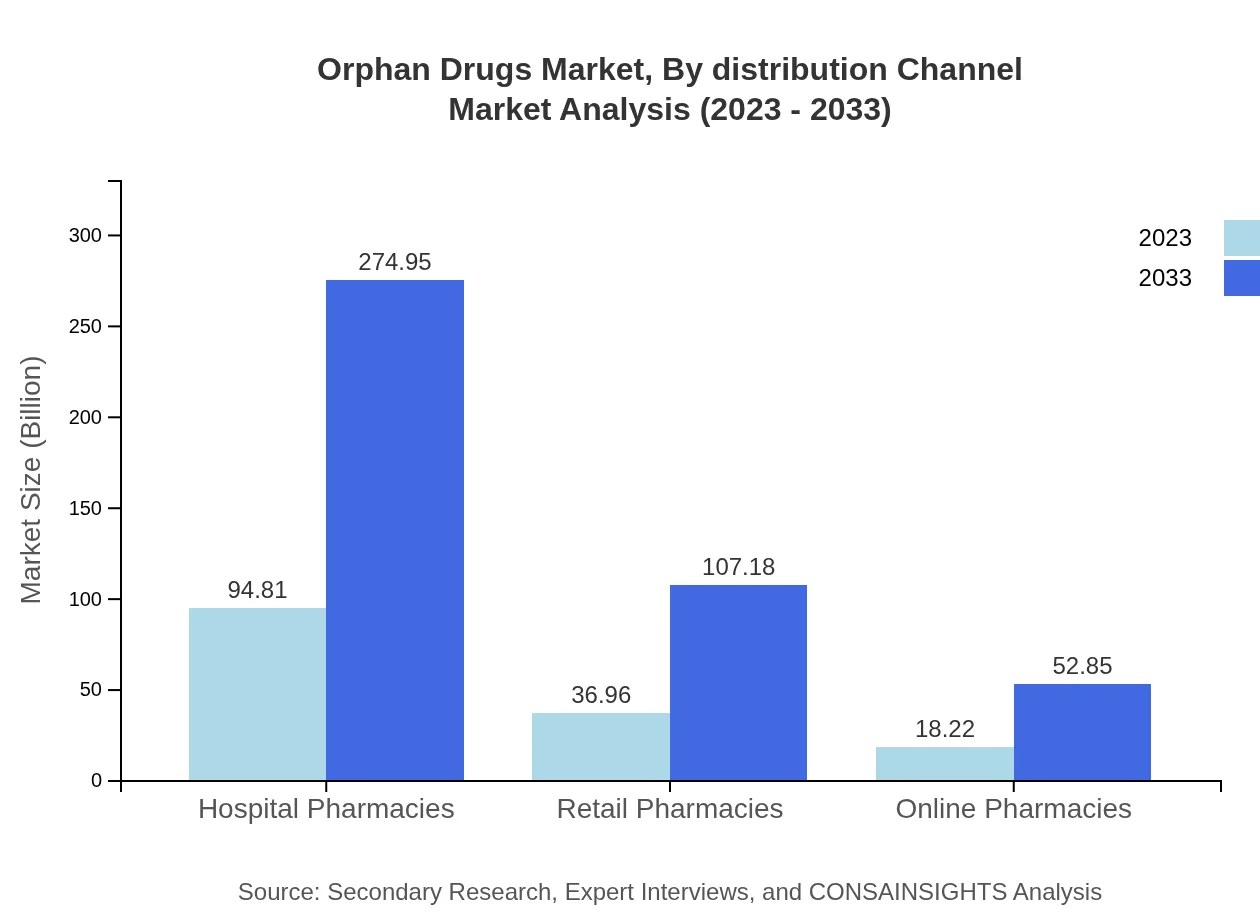
Orphan Drugs Market Analysis By Distribution Channel
In 2023, hospitals were the primary distribution channel, making up a significant volume of orphan drug sales. Specialty clinics also play an essential role, alongside retail and online pharmacies that are becoming increasingly popular for accessing rare treatments.
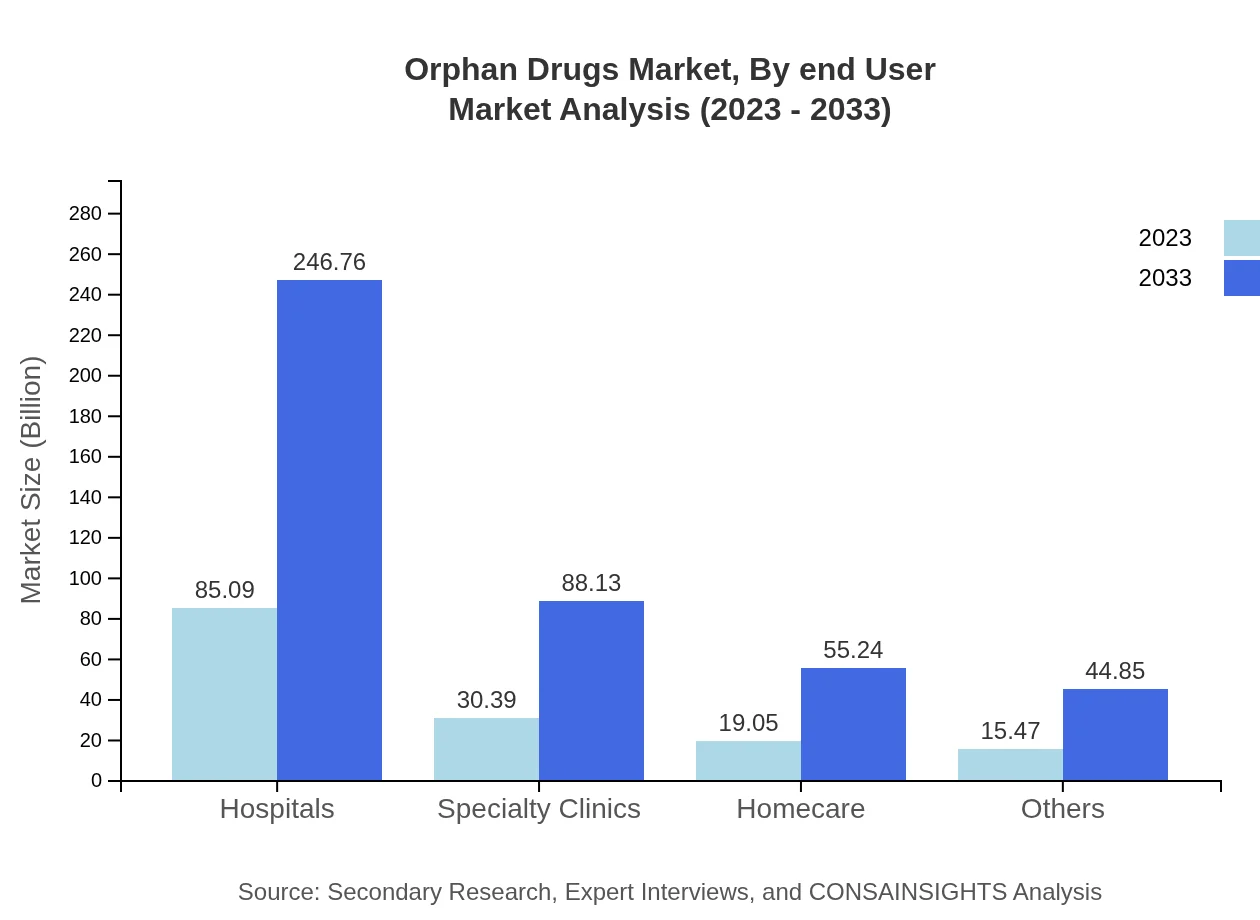
Orphan Drugs Market Analysis By End User
Hospital pharmacies dominated the orphan drugs sector in 2023 due to the intensive treatment requirements for rare diseases. Specialty pharmacies are also growing in importance as they specialize in managing complex therapies and providing necessary patient support.
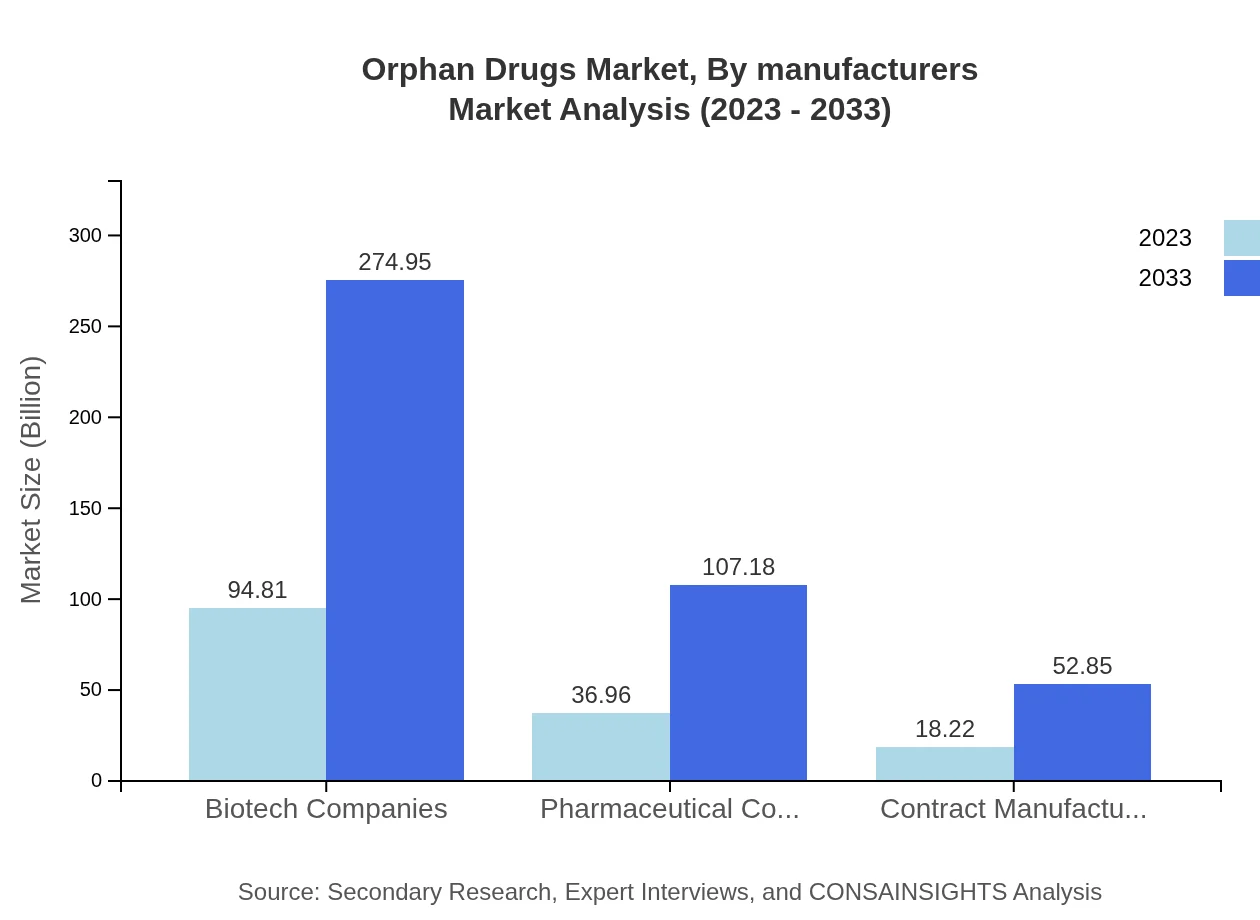
Orphan Drugs Market Analysis By Manufacturers
The orphan drugs market comprises various players, including pharmaceutical giants and specialized biotech companies that focus on rare disease solutions. This sector's competitive landscape continues to evolve with new entrants pursuing innovative treatments.
Orphan Drugs Market Trends and Future Forecast
Tell us your focus area and get a customized research report.
Global Market Leaders and Top Companies in Orphan Drugs Industry
Novartis:
A leading global healthcare company, Novartis focuses on innovative medicines, specifically in the areas of ophthalmology, cardiology, and established therapies for rare diseases.Roche:
Known for its strong position in oncology and personalized medicines, Roche's commitment to rare diseases is evident with a robust pipeline of orphan drug therapies.Sanofi:
Sanofi has a diverse portfolio that encompasses multiple therapeutic areas, including a strong focus on orphan drugs, particularly in hematology and rare genetic diseases.Amgen:
A prominent player in biotechnology, Amgen develops breakthrough therapies for patients suffering from severe illnesses, including those with rare diseases.Bristol-Myers Squibb:
With notable advancements in immuno-oncology, Bristol-Myers Squibb also invests in orphan drugs, focusing on therapies for rare cancers.We're grateful to work with incredible clients.









FAQs
What is the market size of orphan Drugs?
The orphan drugs market is anticipated to reach $150 billion by 2033, growing at a CAGR of 10.8%. This demonstrates significant expansion, driven by the increasing prevalence of rare diseases requiring specialized therapies.
What are the key market players or companies in the orphan Drugs industry?
Key players in the orphan drugs market include leading pharmaceutical and biotech companies. These organizations are focused on innovative drug development to address rare diseases, contributing to market growth.
What are the primary factors driving the growth in the orphan drugs industry?
Growth in the orphan drugs industry is driven by increasing R&D investments, government incentives, and a growing patient population facing rare diseases, all contributing to a competitive market landscape.
Which region is the fastest Growing in the orphan drugs market?
North America is currently the fastest-growing region in the orphan drugs market, projected to reach $155.72 billion by 2033, followed closely by Europe and Asia Pacific, indicating overall robust growth.
Does ConsaInsights provide customized market report data for the orphan drugs industry?
Yes, ConsaInsights offers customized market report data tailored specifically to the orphan drugs industry, allowing stakeholders to gain insights unique to their business needs.
What deliverables can I expect from this orphan drugs market research project?
Deliverables from the orphan drugs market research project include comprehensive market analysis, regional breakdowns, segment insights, and competitive landscape assessments, aiding strategic planning.
What are the market trends of orphan drugs?
Current trends in the orphan drugs market include increasing collaboration among companies, a rise in personalized medicine approaches, and advancing regulatory frameworks, all shaping the future of this sector.