Orthopedic Regenerative Surgical Products Market Report
Published Date: 31 January 2026 | Report Code: orthopedic-regenerative-surgical-products
Orthopedic Regenerative Surgical Products Market Size, Share, Industry Trends and Forecast to 2033
This report provides comprehensive insights into the Orthopedic Regenerative Surgical Products market, focusing on market size, regional analysis, key players, and future forecasts from 2023 to 2033.
| Metric | Value |
|---|---|
| Study Period | 2023 - 2033 |
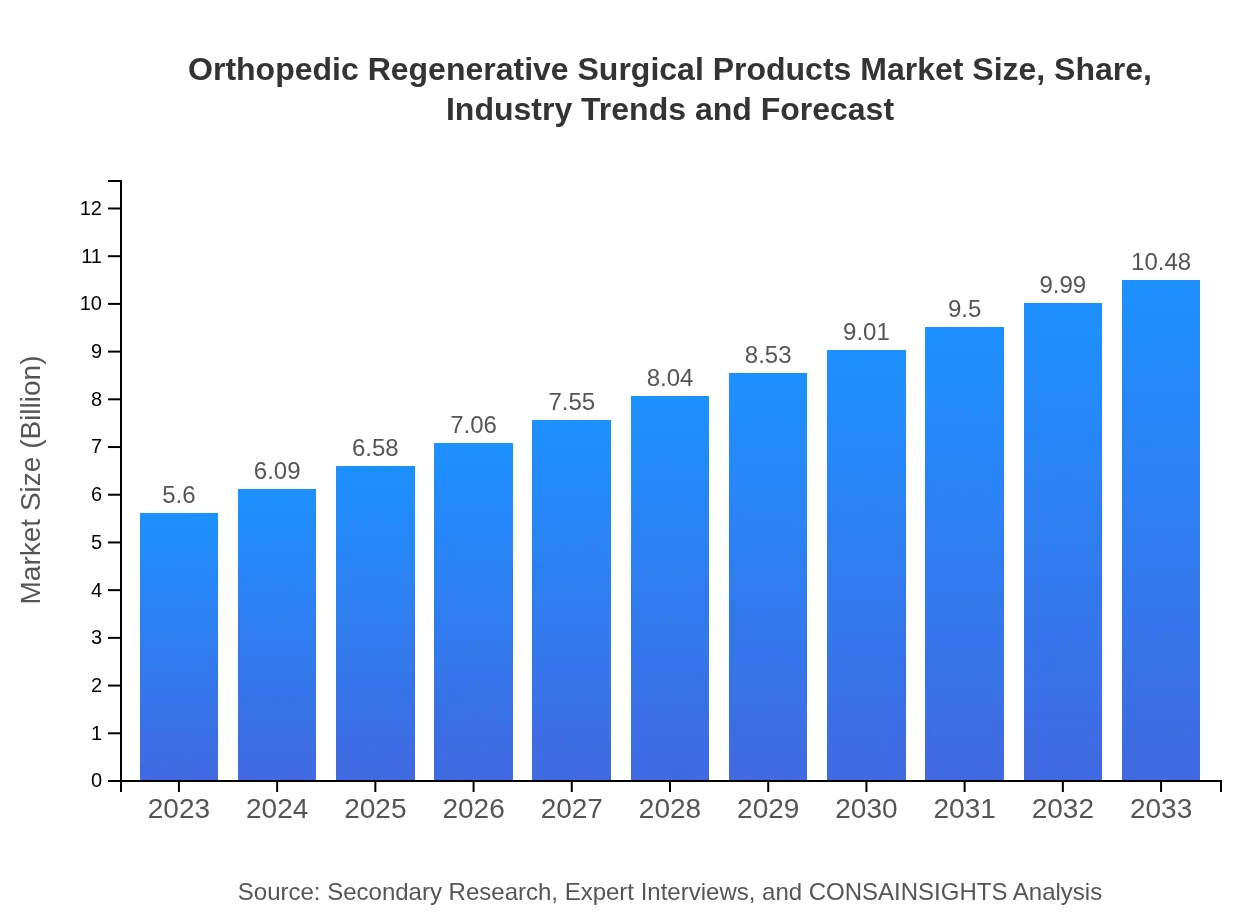
| 2023 Market Size | $5.60 Billion |
| CAGR (2023-2033) | 6.3% |
| 2033 Market Size | $10.48 Billion |
| Top Companies | Medtronic , Zimmer Biomet, Stryker Corporation, Exactech |
| Last Modified Date | 31 January 2026 |
Orthopedic Regenerative Surgical Products Market Overview
Customize Orthopedic Regenerative Surgical Products Market Report market research report
- ✔ Get in-depth analysis of Orthopedic Regenerative Surgical Products market size, growth, and forecasts.
- ✔ Understand Orthopedic Regenerative Surgical Products's regional dynamics and industry-specific trends.
- ✔ Identify potential applications, end-user demand, and growth segments in Orthopedic Regenerative Surgical Products
What is the Market Size & CAGR of Orthopedic Regenerative Surgical Products market in 2023?
Orthopedic Regenerative Surgical Products Industry Analysis
Orthopedic Regenerative Surgical Products Market Segmentation and Scope
Tell us your focus area and get a customized research report.
Orthopedic Regenerative Surgical Products Market Analysis Report by Region
Europe Orthopedic Regenerative Surgical Products Market Report:
The European market for orthopedic regenerative surgical products is expected to grow from $1.59 billion in 2023 to $2.97 billion by 2033. Factors influencing this growth include stringent regulations promoting product safety and effectiveness and a strong focus on research in regenerative medicine.Asia Pacific Orthopedic Regenerative Surgical Products Market Report:
In the Asia Pacific region, the market size is anticipated to grow from $1.08 billion in 2023 to $2.03 billion by 2033. Key drivers include rapid urbanization, increased healthcare expenditure, and a burgeoning population increasingly prone to orthopedic injuries.North America Orthopedic Regenerative Surgical Products Market Report:
North America leads the market, estimated at $1.98 billion in 2023, expanding to $3.70 billion by 2033. The region's growth is fueled by high healthcare expenditures, advanced medical facilities, and a high prevalence of orthopedic disorders.South America Orthopedic Regenerative Surgical Products Market Report:
South America is projected to grow from $0.46 billion in 2023 to $0.86 billion by 2033. The growth is supported by improving healthcare infrastructure and rising awareness about advanced orthopedic procedures.Middle East & Africa Orthopedic Regenerative Surgical Products Market Report:
The Middle East and Africa region are forecasted to grow from $0.49 billion in 2023 to $0.92 billion by 2033. The market will benefit from rising healthcare investment and increasing healthcare access in emerging economies.Tell us your focus area and get a customized research report.
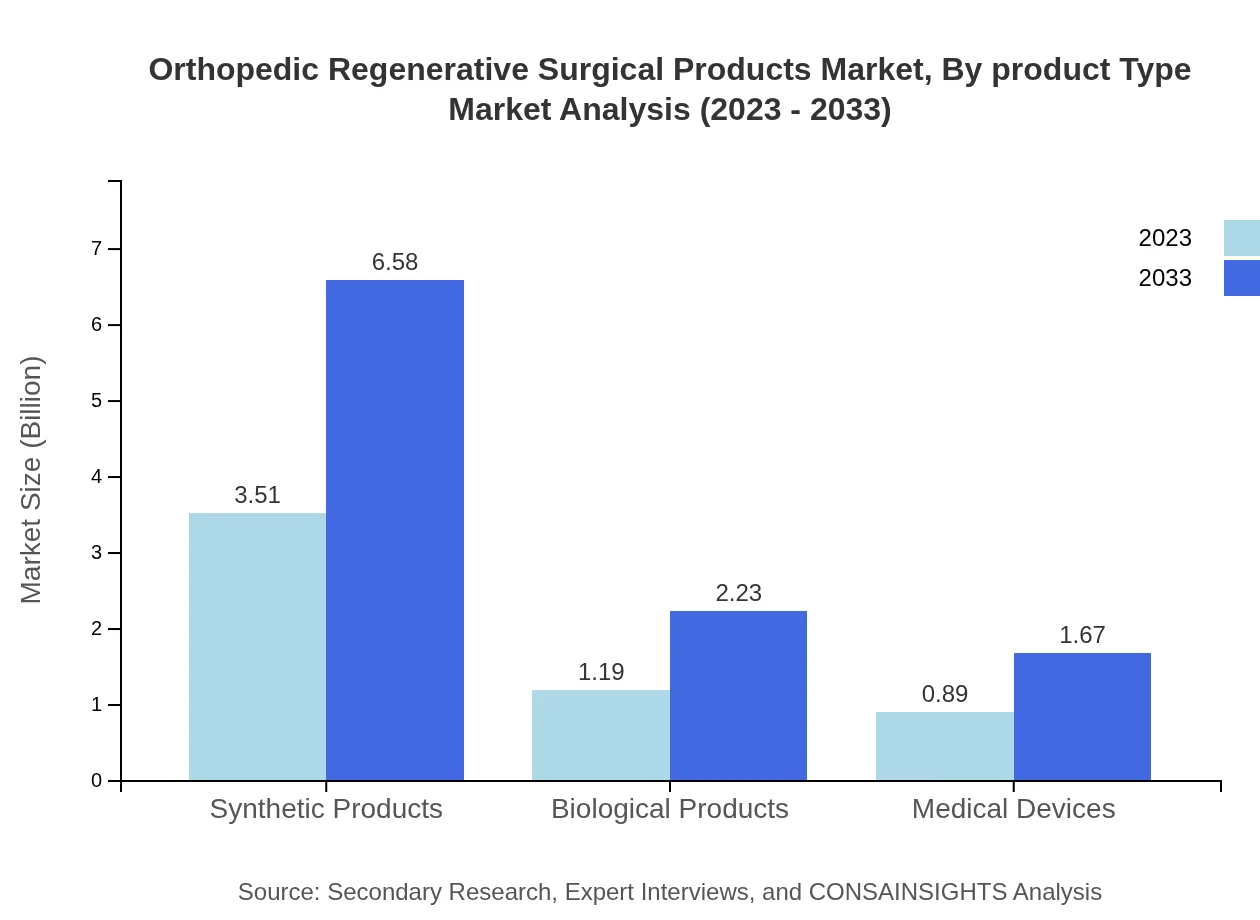
Orthopedic Regenerative Surgical Products Market Analysis By Product Type
The market consists of various product types including Cell-based Therapies, Gene Therapy, and Tissue Engineering. Cell-based therapies dominate the market, expected to reach $6.58 billion by 2033, holding a 62.75% share. Gene therapy showcases substantial growth, moving from $1.19 billion currently to $2.23 billion by 2033.
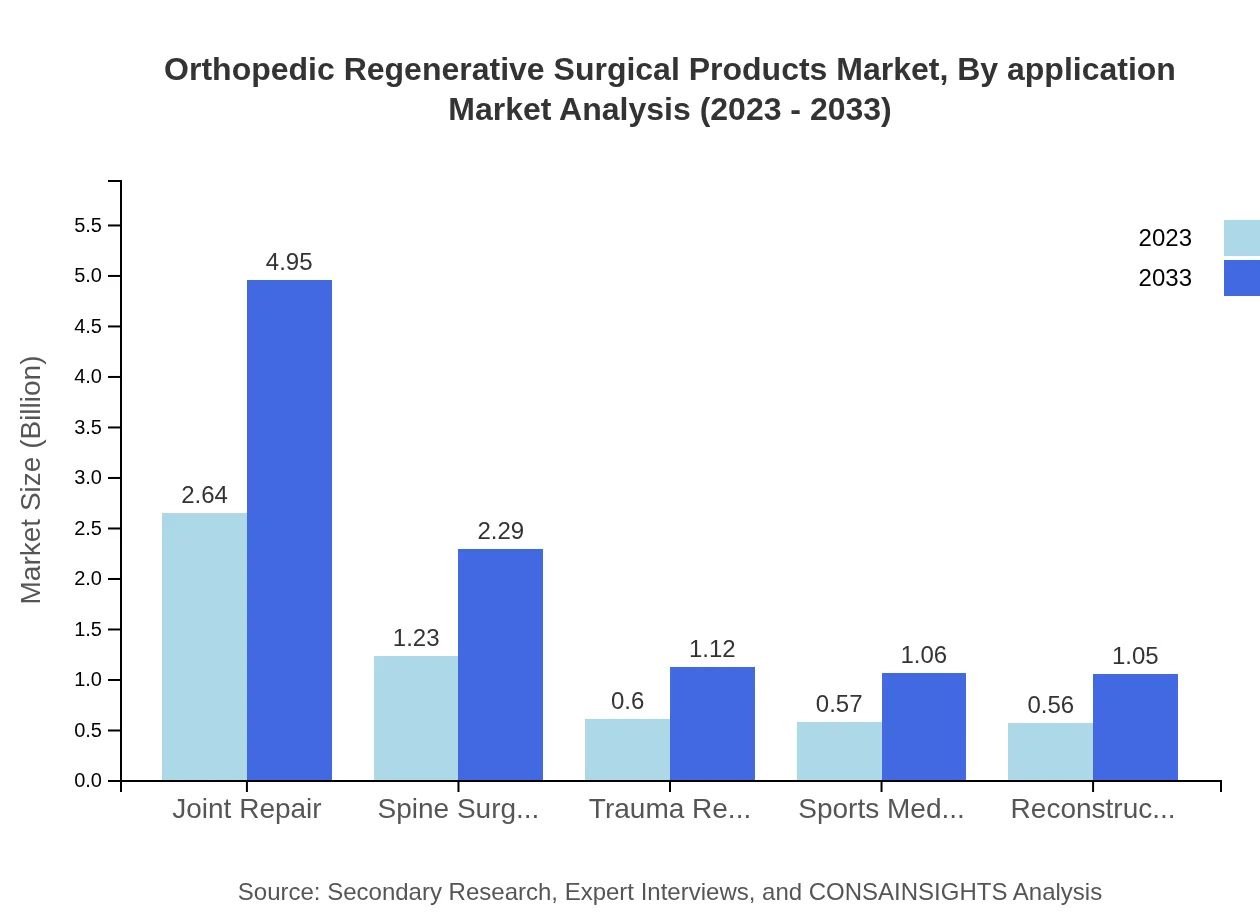
Orthopedic Regenerative Surgical Products Market Analysis By Application
Applications of orthopedic regenerative products span Joint Repair, Spine Surgery, and Trauma Repair. Joint repair holds a significant market share, projected at $4.95 billion by 2033, with spine surgery accommodating increasing demand for innovative solutions.
Orthopedic Regenerative Surgical Products Market Analysis By End User
End-users include Hospitals, Ambulatory Surgical Centers, and Orthopedic Clinics, where Hospitals account for a majority market share, projected to expand to $6.08 billion by 2033, attributing to increased surgical procedures conducted in these settings.
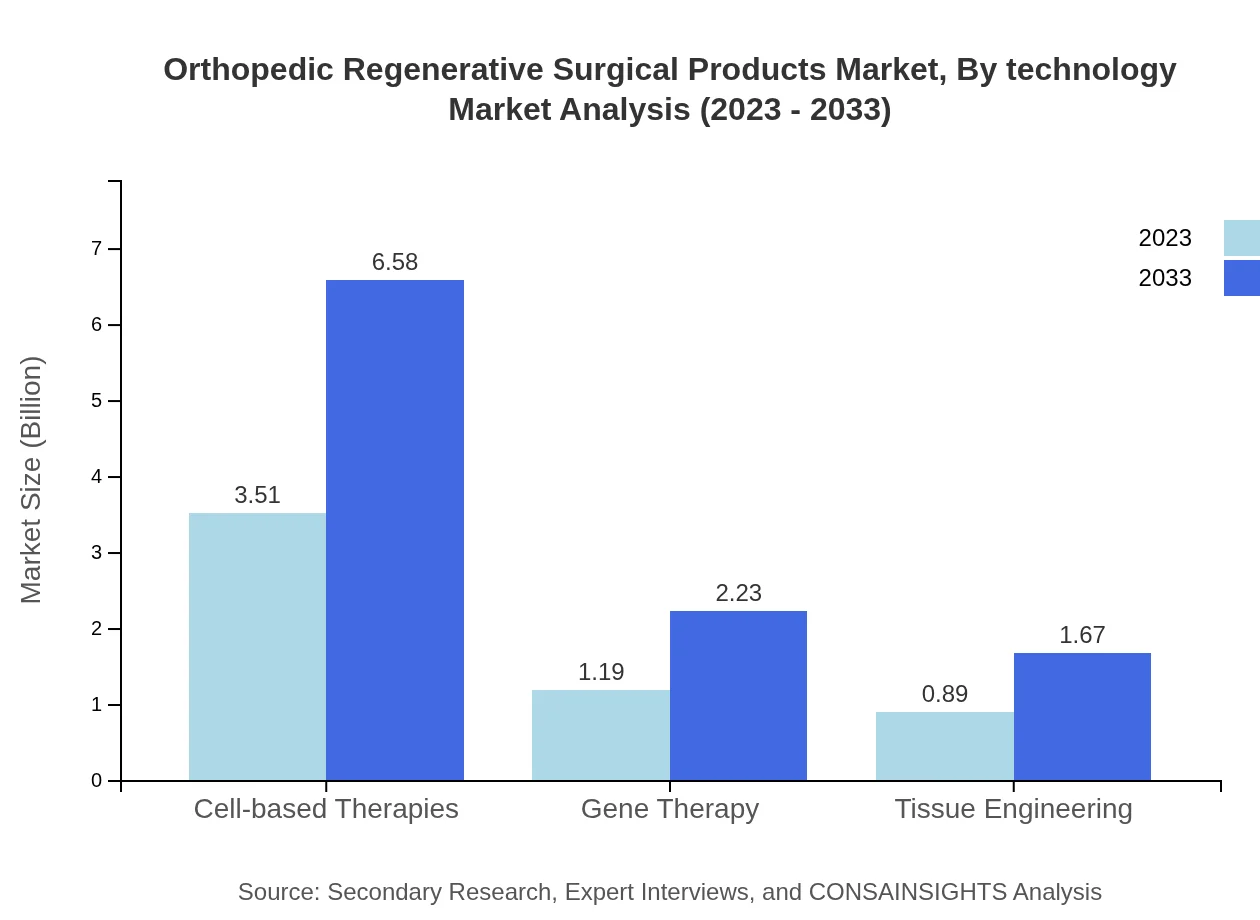
Orthopedic Regenerative Surgical Products Market Analysis By Technology
Technological advancements play a crucial role in driving the orthopedic regenerative products market, with digital solutions enhancing surgical precision and patient outcomes. Emerging technologies are expected to propel product innovation in R&D settings.
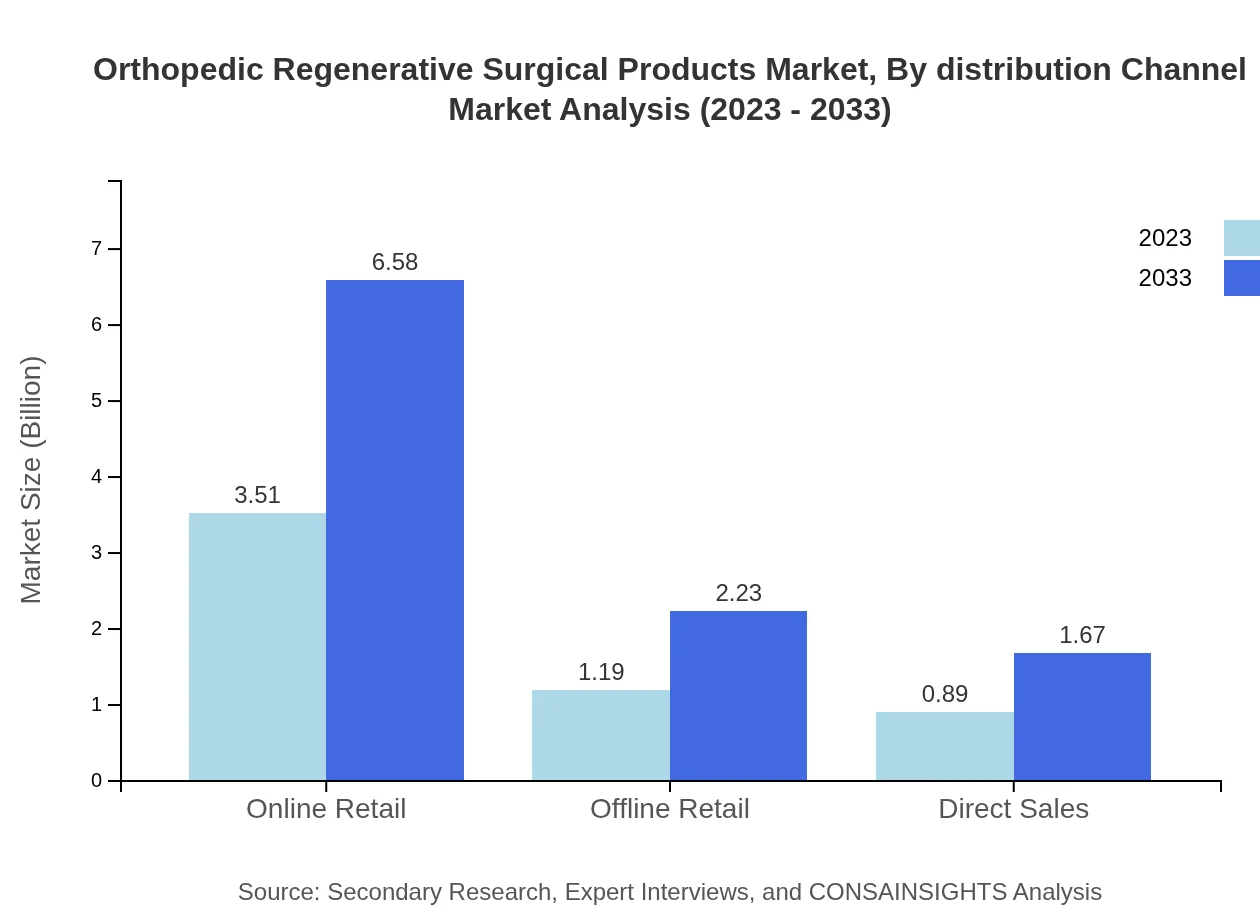
Orthopedic Regenerative Surgical Products Market Analysis By Distribution Channel
The market is served through Online Retail, Offline Retail, and Direct Sales channels, with Online Retail emerging as the dominant channel. It is projected to reach $6.58 billion by 2033, driven by increasing e-commerce adoption.
Orthopedic Regenerative Surgical Products Market Trends and Future Forecast
Tell us your focus area and get a customized research report.
Global Market Leaders and Top Companies in Orthopedic Regenerative Surgical Products Industry
Medtronic :
Medtronic is a global leader in medical technology, supporting orthopedic procedures with innovative regenerative surgical products that enhance recovery.Zimmer Biomet:
Zimmer Biomet offers a broad portfolio of orthopedic solutions, focusing on regenerative treatments that optimize patient outcomes and support joint health.Stryker Corporation:
Stryker specializes in regenerative product development across orthopedic fields, emphasizing on research-backed advancements that address complex surgical challenges.Exactech:
Exactech provides innovative and advanced orthopedic surgical solutions, particularly in joint replacement and cartilage repair technologies.We're grateful to work with incredible clients.









FAQs
What is the market size of orthopedic Regenerative Surgical Products?
The orthopedic regenerative surgical products market is valued at approximately $5.6 billion in 2023 and is projected to grow at a CAGR of 6.3% over the next decade, indicating robust expansion and increasing demand for innovative healthcare solutions.
What are the key market players or companies in this orthopedic Regenerative Surgical Products industry?
Key players in the orthopedic regenerative surgical products market include renowned companies such as DePuy Synthes, Stryker, Medtronic, and Smith & Nephew. These companies are heavily involved in research and product development to maintain competitive advantages.
What are the primary factors driving the growth in the orthopedic Regenerative Surgical Products industry?
Factors driving growth include an aging population requiring orthopedic care, advancements in regenerative technologies, increased sports injuries, and a surge in minimally invasive surgical procedures, making regenerative products more attractive.
Which region is the fastest Growing in the orthopedic Regenerative Surgical Products market?
The regions projected to grow the fastest are North America, with a market size expected to increase from $1.98 billion in 2023 to $3.70 billion by 2033, and Europe, growing from $1.59 billion to $2.97 billion in the same period.
Does ConsaInsights provide customized market report data for the orthopedic Regenerative Surgical Products industry?
Yes, ConsaInsights specializes in offering customized market reports tailored to clients' specific needs, providing detailed insights and tailor-made data on the orthopedic regenerative surgical products industry's trends and forecasts.
What deliverables can I expect from this orthopedic Regenerative Surgical Products market research project?
Deliverables include comprehensive market analysis reports, detailed segmentations, forecasts, competitive landscape overviews, and actionable insights to support strategic decision-making in the orthopedic regenerative surgical products field.
What are the market trends of orthopedic Regenerative Surgical Products?
Trends include an increasing shift towards minimally invasive techniques, a rise in cell-based and gene therapies, growth in online sales channels, and enhanced focus on personalized treatment plans, reflecting innovations in the industry.