Ovarian Cancer Diagnostics And Therapeutics Market Report
Published Date: 31 January 2026 | Report Code: ovarian-cancer-diagnostics-and-therapeutics
Ovarian Cancer Diagnostics And Therapeutics Market Size, Share, Industry Trends and Forecast to 2033
This report provides a comprehensive analysis of the Ovarian Cancer Diagnostics and Therapeutics market, including market size, trends, forecasts (2023-2033), and insights on advancements in technology and product performance.
| Metric | Value |
|---|---|
| Study Period | 2023 - 2033 |
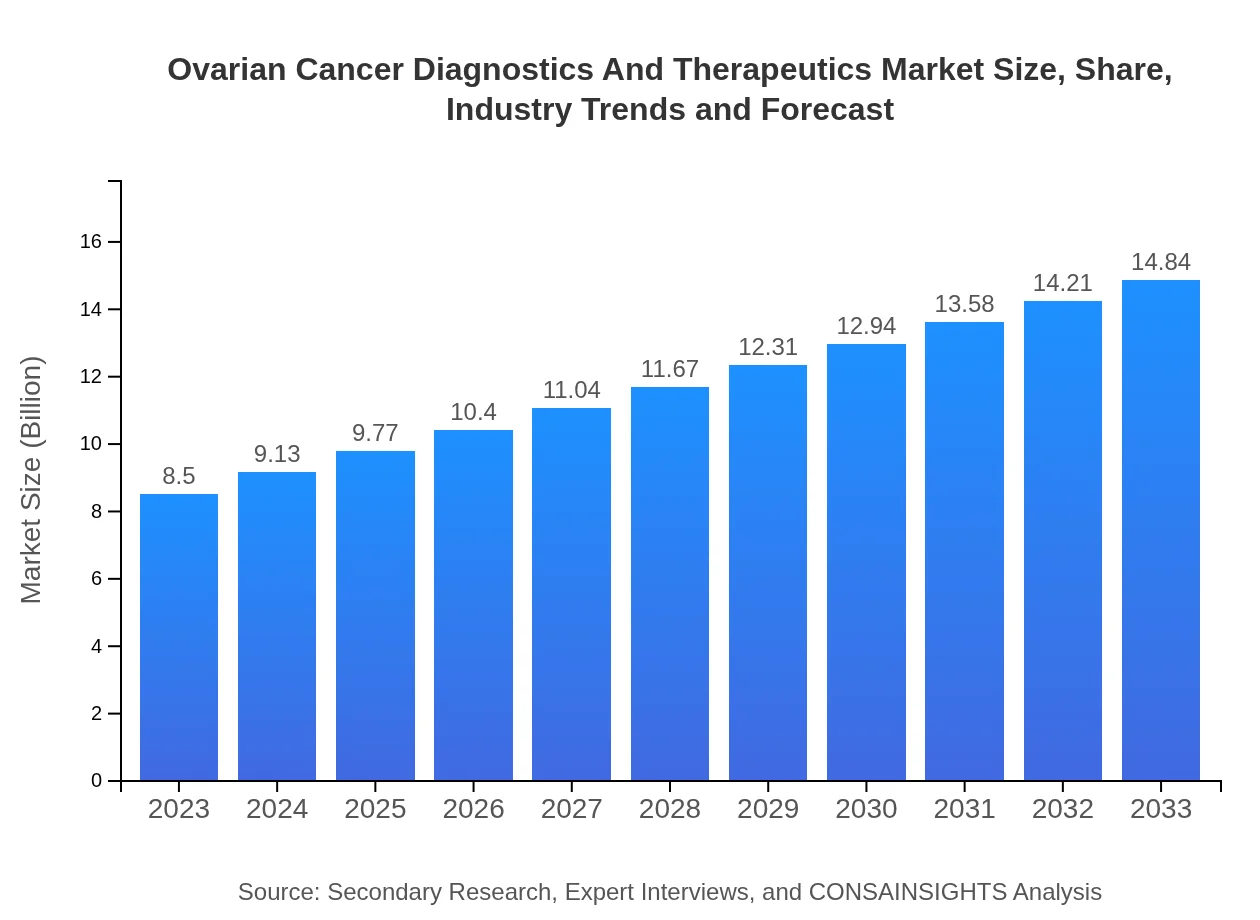
| 2023 Market Size | $8.50 Billion |
| CAGR (2023-2033) | 5.6% |
| 2033 Market Size | $14.84 Billion |
| Top Companies | Roche, Bristol-Myers Squibb, Merck & Co., AbbVie, Amgen |
| Last Modified Date | 31 January 2026 |
Ovarian Cancer Diagnostics And Therapeutics Market Overview
Customize Ovarian Cancer Diagnostics And Therapeutics Market Report market research report
- ✔ Get in-depth analysis of Ovarian Cancer Diagnostics And Therapeutics market size, growth, and forecasts.
- ✔ Understand Ovarian Cancer Diagnostics And Therapeutics's regional dynamics and industry-specific trends.
- ✔ Identify potential applications, end-user demand, and growth segments in Ovarian Cancer Diagnostics And Therapeutics
What is the Market Size & CAGR of Ovarian Cancer Diagnostics And Therapeutics market in 2023 and 2033?
Ovarian Cancer Diagnostics And Therapeutics Industry Analysis
Ovarian Cancer Diagnostics And Therapeutics Market Segmentation and Scope
Tell us your focus area and get a customized research report.
Ovarian Cancer Diagnostics And Therapeutics Market Analysis Report by Region
Europe Ovarian Cancer Diagnostics And Therapeutics Market Report:
Europe’s market is valued at $2.36 billion in 2023, expected to reach $4.12 billion by 2033. The region shows strong growth due to advanced healthcare systems, high awareness levels, and supportive policies for cancer care.Asia Pacific Ovarian Cancer Diagnostics And Therapeutics Market Report:
The Asia Pacific region is projected to experience significant growth in the ovarian cancer diagnostics market, with a 2023 valuation of $1.65 billion, anticipated to rise to $2.88 billion by 2033. This growth is driven by increasing healthcare expenditure, rising awareness, and a growing patient population.North America Ovarian Cancer Diagnostics And Therapeutics Market Report:
North America holds the largest market share, valued at $3.18 billion in 2023, projected to grow to $5.56 billion by 2033. Leading in R&D and advanced healthcare facilities, this region benefits from robust government initiatives and significant investments in oncology research.South America Ovarian Cancer Diagnostics And Therapeutics Market Report:
In South America, the ovarian cancer diagnostics market is relatively smaller, valued at $0.48 billion in 2023 and estimated to reach $0.84 billion by 2033. The region faces challenges including healthcare access and resource constraints but is witnessing gradual improvements in awareness and treatment options.Middle East & Africa Ovarian Cancer Diagnostics And Therapeutics Market Report:
The Middle East and Africa exhibit a market valued at $0.82 billion in 2023, projected to grow to $1.44 billion by 2033, driven by increasing investments in healthcare infrastructure and growing awareness regarding cancer diagnostics.Tell us your focus area and get a customized research report.
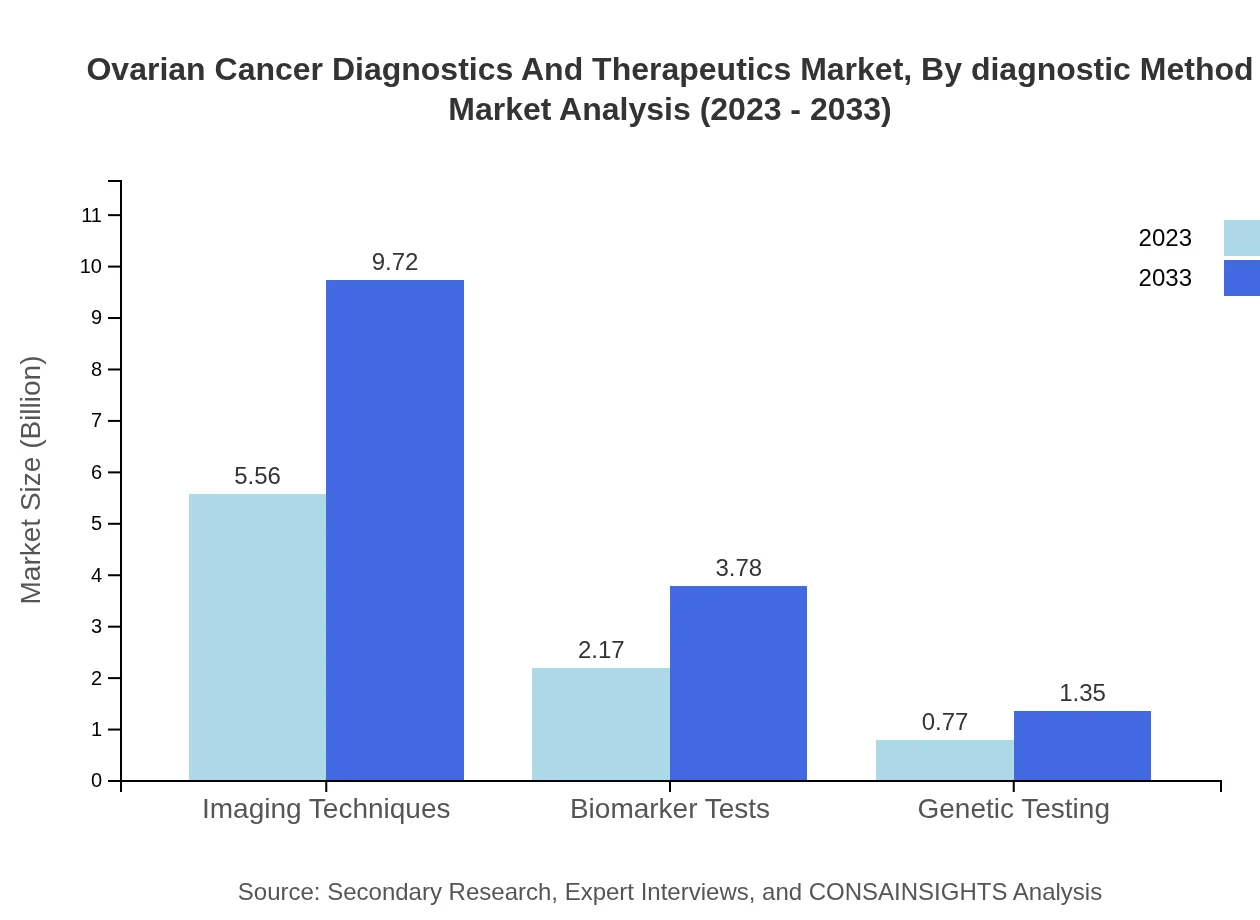
Ovarian Cancer Diagnostics And Therapeutics Market Analysis By Diagnostic Method
The ovarian cancer diagnostics market is primarily segmented into imaging techniques and biomarker tests. Imaging techniques accounted for approximately $5.56 billion in 2023 and are expected to reach $9.72 billion by 2033, holding a significant share of 65.45%. Biomarker tests are projected to grow from $2.17 billion in 2023 to $3.78 billion by 2033, capturing 25.48% of the diagnostic method market.
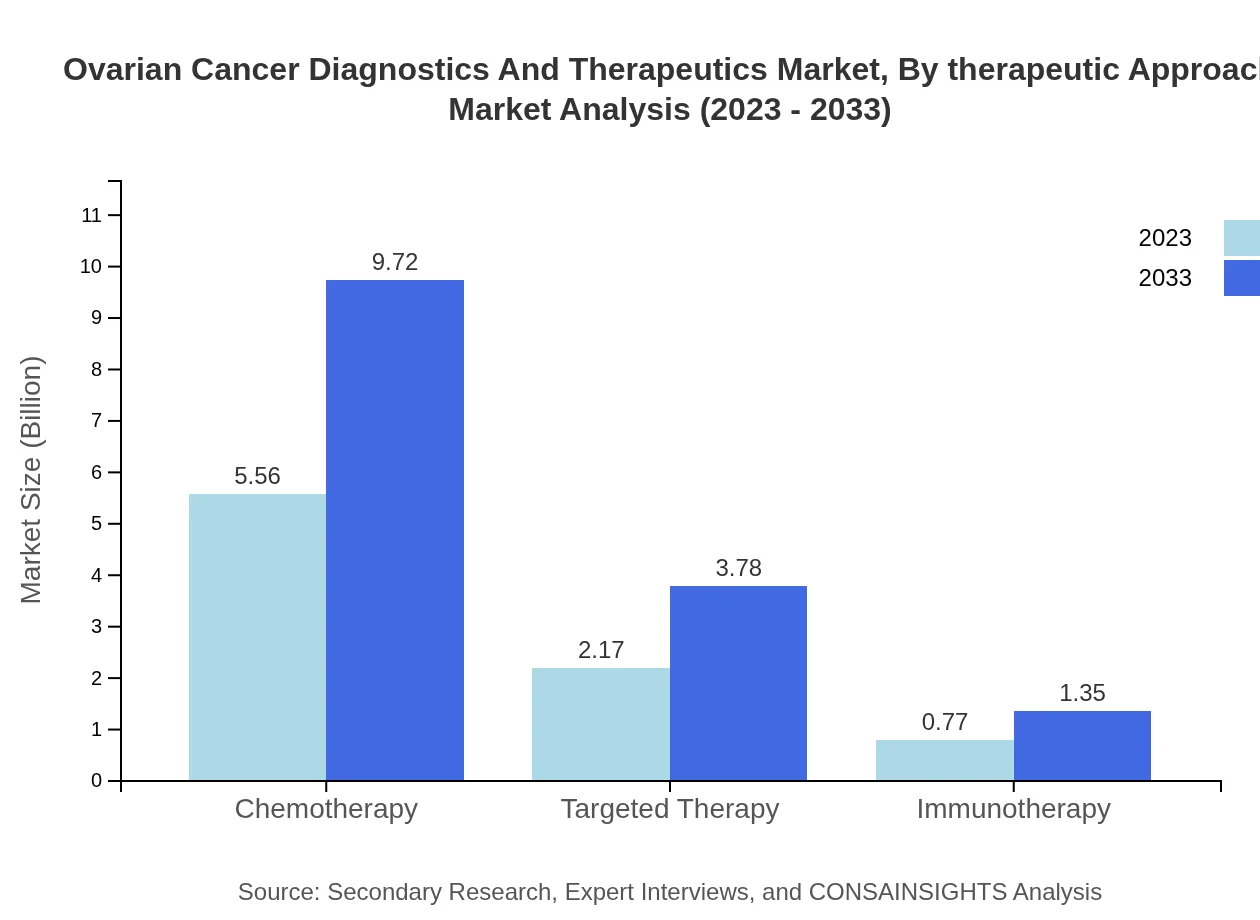
Ovarian Cancer Diagnostics And Therapeutics Market Analysis By Therapeutic Approach
The therapeutic market segment reflects a similar growth pattern, with chemotherapy leading at $5.56 billion in 2023 and expected to rise to $9.72 billion by 2033, retaining a substantial share of 65.45%. Targeted therapy and immunotherapy are also significant, projected to grow from $2.17 billion to $3.78 billion and $0.77 billion to $1.35 billion respectively over the same period.
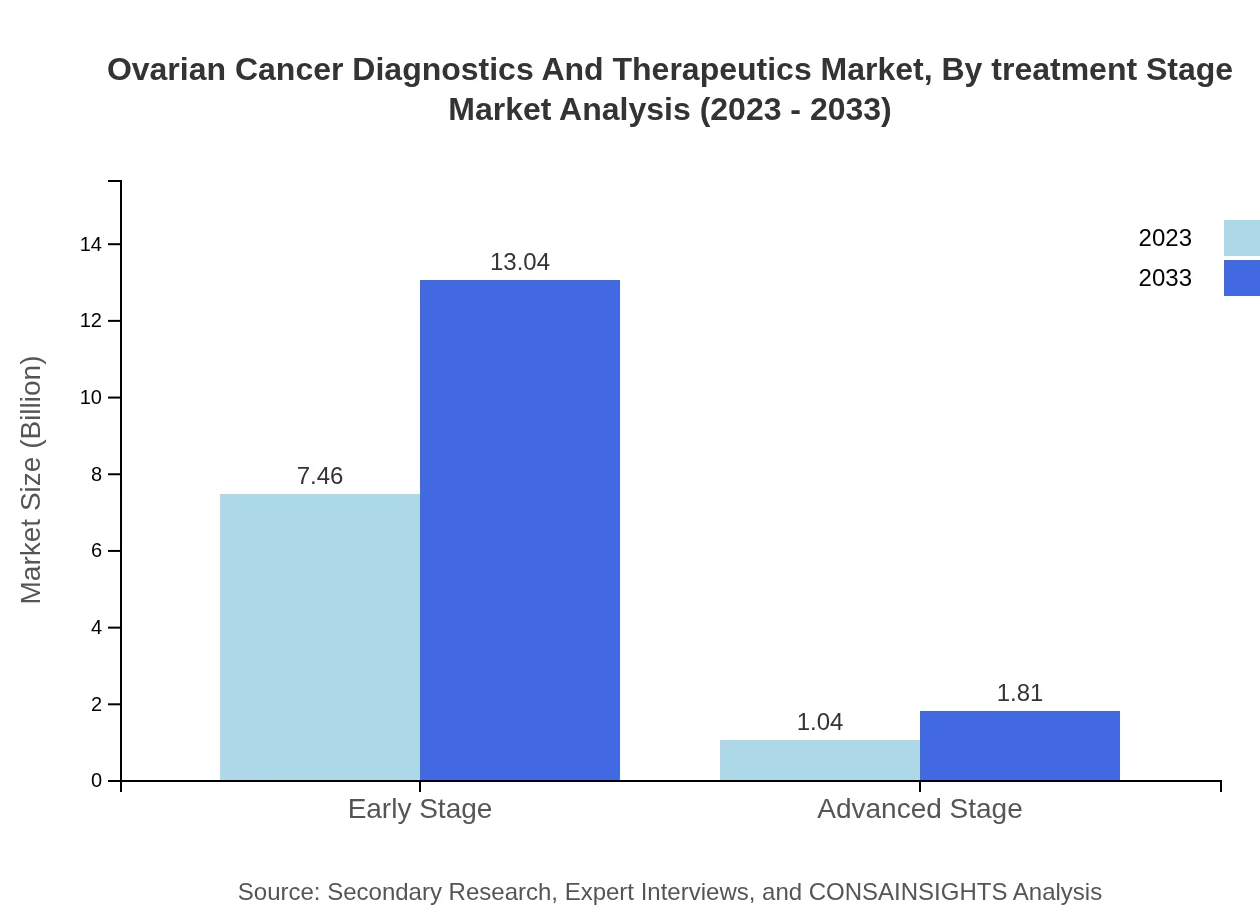
Ovarian Cancer Diagnostics And Therapeutics Market Analysis By Treatment Stage
The market is divided into early-stage and advanced-stage treatments, with early-stage diagnosis commanding $7.46 billion in 2023, slated to grow to $13.04 billion by 2033, accounting for 87.82% of the treatment stage market. Advanced-stage treatments represent a smaller but growing market, increasing from $1.04 billion to $1.81 billion.
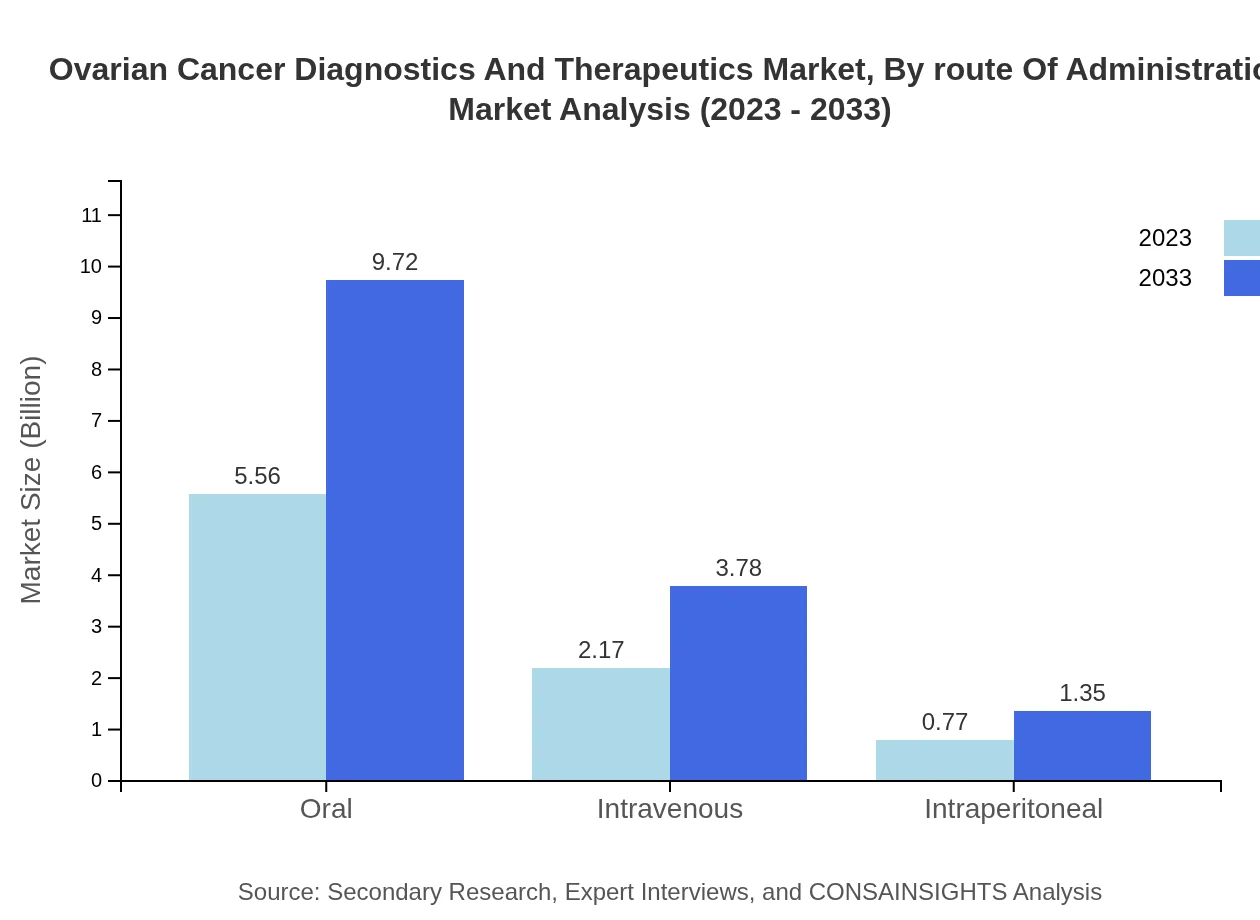
Ovarian Cancer Diagnostics And Therapeutics Market Analysis By Route Of Administration
In terms of routes of administration, oral medications dominate the market, valued at $5.56 billion in 2023 and projected to reach $9.72 billion by 2033, constituting 65.45% of the segment. Intravenous and intraperitoneal routes are also relevant, with increasing use projected as therapeutic advancements continue.
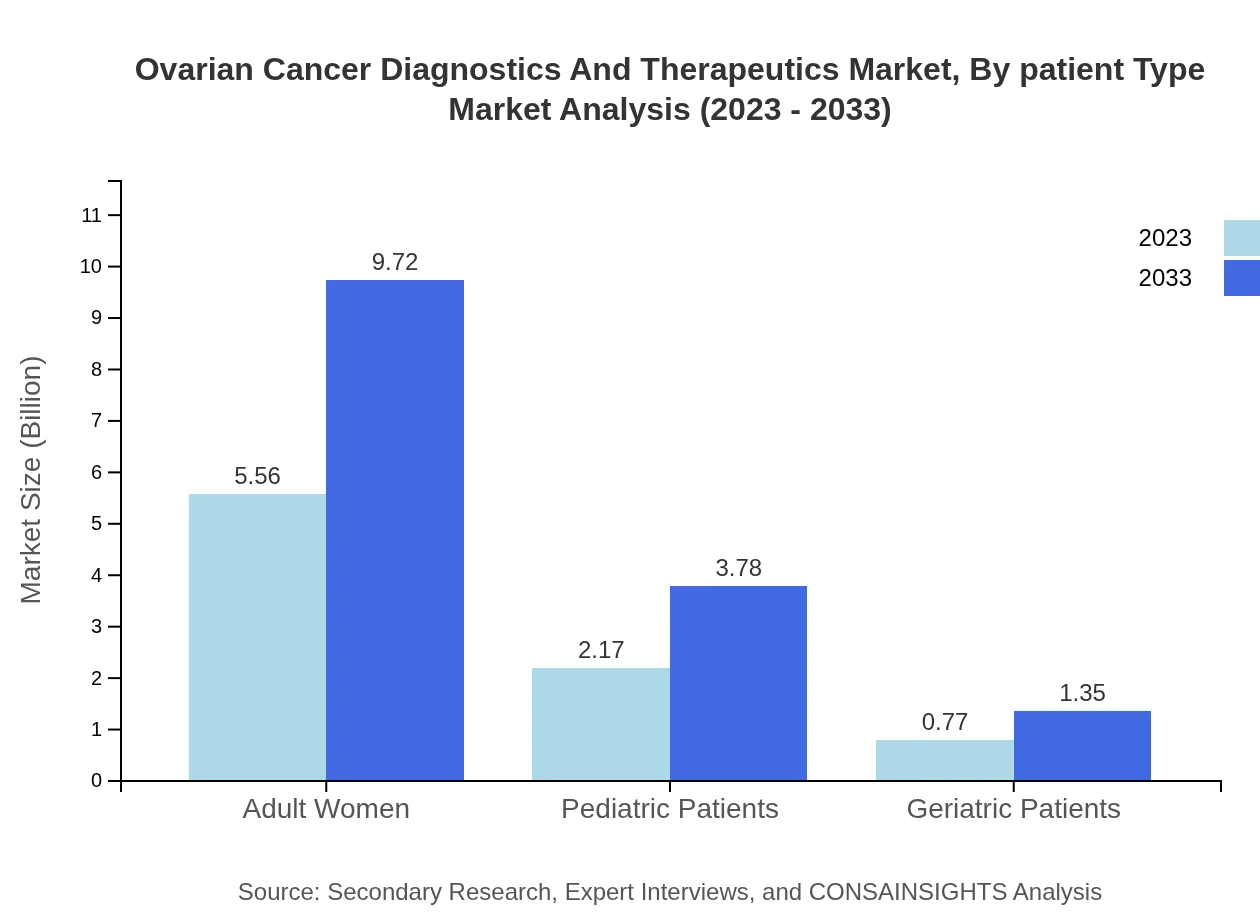
Ovarian Cancer Diagnostics And Therapeutics Market Analysis By Patient Type
The market for ovarian cancer therapeutics is segmented based on patient types. Adult women form the largest segment with a market size of $5.56 billion in 2023, expected to grow to $9.72 billion by 2033. Pediatric and geriatric patients represent niches with projections of $2.17 billion and $0.77 billion respectively in 2023, expanding as diagnostic and therapeutic methodologies improve.
Ovarian Cancer Diagnostics And Therapeutics Market Trends and Future Forecast
Tell us your focus area and get a customized research report.
Global Market Leaders and Top Companies in Ovarian Cancer Diagnostics And Therapeutics Industry
Roche:
A leader in oncology diagnostics, Roche develops innovative tests and therapeutics aimed at improving patient outcomes in ovarian cancer.Bristol-Myers Squibb:
Known for its advanced immunotherapy solutions, Bristol-Myers Squibb plays a critical role in the treatment landscape of ovarian cancer.Merck & Co.:
Merck is recognized for its development of targeted therapies and significant contributions to clinical trials in ovarian cancer therapeutics.AbbVie:
AbbVie is a key player specializing in innovative cancer treatments, focusing on personalized medicine for ovarian cancer patients.Amgen:
With a commitment to research and development, Amgen is at the forefront of creating breakthrough therapies for ovarian cancer.We're grateful to work with incredible clients.









FAQs
What is the market size of ovarian Cancer Diagnostics And Therapeutics?
The ovarian cancer diagnostics and therapeutics market is projected to be valued at approximately $8.5 billion in 2023 and is expected to grow at a CAGR of 5.6% over the next decade.
What are the key market players or companies in the ovarian Cancer Diagnostics And Therapeutics industry?
Key players in the ovarian cancer diagnostics and therapeutics market include major pharmaceutical companies and diagnostic firms. These companies focus on innovative treatments, genetic testing, biomarker identification, and advanced imaging technologies.
What are the primary factors driving the growth in the ovarian Cancer Diagnostics And Therapeutics industry?
Growth in the ovarian cancer diagnostics and therapeutics market is driven by increasing awareness of cancer treatment options, advancements in diagnostic technologies, rising incidence rates of ovarian cancer, and more targeted and personalized therapy approaches.
Which region is the fastest Growing in the ovarian Cancer Diagnostics And Therapeutics?
The fastest-growing region in the ovarian cancer diagnostics and therapeutics market is North America, projected to increase from $3.18 billion in 2023 to $5.56 billion by 2033, driven by innovative research and high healthcare spending.
Does ConsaInsights provide customized market report data for the ovarian Cancer Diagnostics And Therapeutics industry?
Yes, ConsaInsights offers customized market report data tailored to specific client needs in the ovarian cancer diagnostics and therapeutics industry, ensuring comprehensive insights and strategic guidance.
What deliverables can I expect from this ovarian Cancer Diagnostics And Therapeutics market research project?
Deliverables from this market research project will include detailed reports on market trends, regional insights, competitive analysis, segmentation data, and actionable recommendations based on the latest industry developments.
What are the market trends of ovarian Cancer Diagnostics And Therapeutics?
Current trends in the ovarian cancer diagnostics and therapeutics market include a shift towards personalized medicine, advancements in biomarker testing, integration of AI in diagnostics, and the growing importance of early detection strategies.