Paclitaxeleluting Stent Market Report
Published Date: 31 January 2026 | Report Code: paclitaxeleluting-stent
Paclitaxeleluting Stent Market Size, Share, Industry Trends and Forecast to 2033
This report explores the Paclitaxeleluting Stent market, providing insights into market dynamics, industry trends, technology advancements, regional analysis, and a detailed forecast for the period 2023-2033.
| Metric | Value |
|---|---|
| Study Period | 2023 - 2033 |
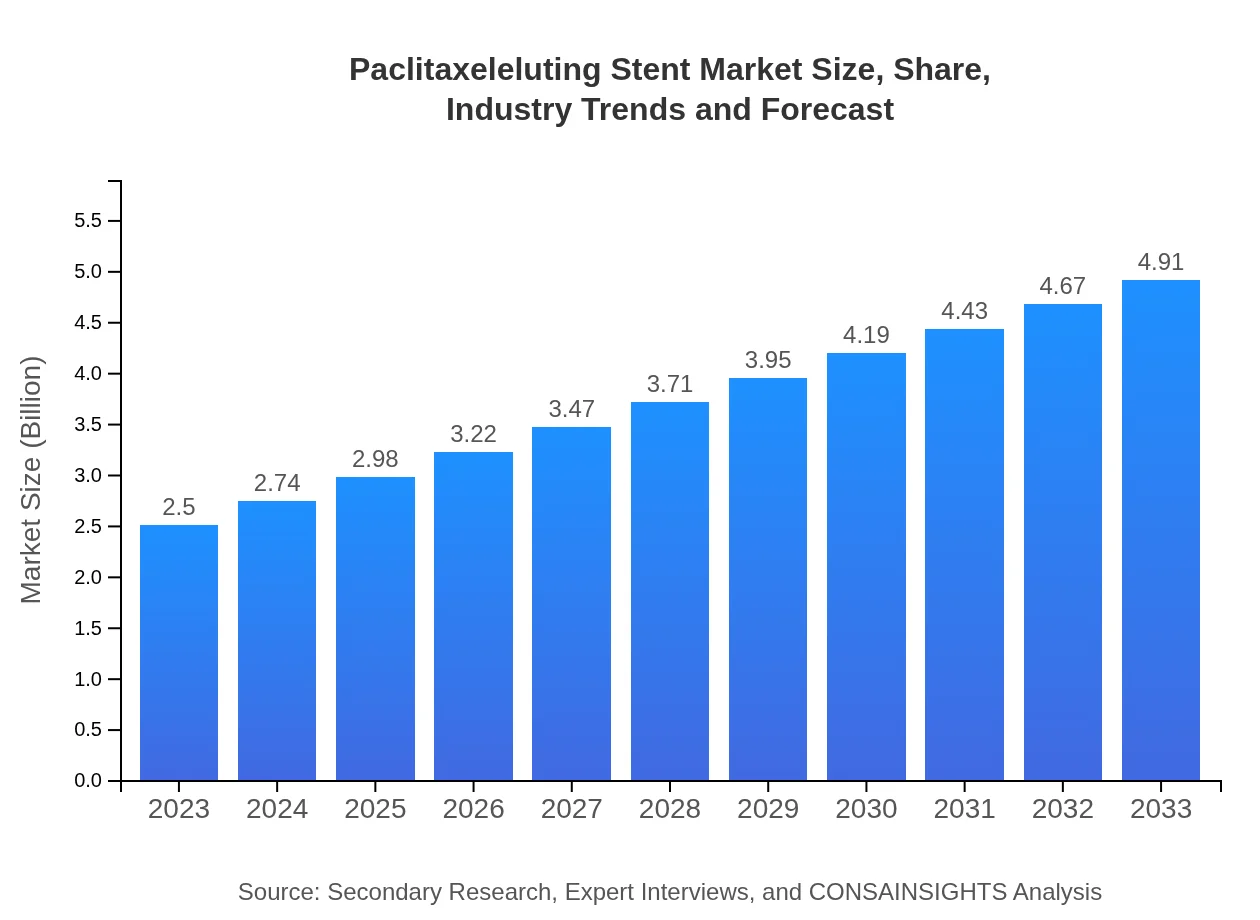
| 2023 Market Size | $2.50 Billion |
| CAGR (2023-2033) | 6.8% |
| 2033 Market Size | $4.91 Billion |
| Top Companies | Medtronic , Boston Scientific, Abbott Laboratories, B. Braun Melsungen AG |
| Last Modified Date | 31 January 2026 |
Paclitaxeleluting Stent Market Overview
Customize Paclitaxeleluting Stent Market Report market research report
- ✔ Get in-depth analysis of Paclitaxeleluting Stent market size, growth, and forecasts.
- ✔ Understand Paclitaxeleluting Stent's regional dynamics and industry-specific trends.
- ✔ Identify potential applications, end-user demand, and growth segments in Paclitaxeleluting Stent
What is the Market Size & CAGR of the Paclitaxeleluting Stent market in 2023?
Paclitaxeleluting Stent Industry Analysis
Paclitaxeleluting Stent Market Segmentation and Scope
Tell us your focus area and get a customized research report.
Paclitaxeleluting Stent Market Analysis Report by Region
Europe Paclitaxeleluting Stent Market Report:
In Europe, the Paclitaxeleluting Stent market was valued at approximately $0.83 billion in 2023, expected to increase to $1.62 billion by 2033. The market is pushed by robust regulatory frameworks and rising geriatric populations requiring enhanced cardiovascular interventions.Asia Pacific Paclitaxeleluting Stent Market Report:
In the Asia Pacific region, the Paclitaxeleluting Stent market was valued at approximately $0.45 billion in 2023, projected to double to $0.88 billion by 2033. Increasing healthcare infrastructure, growing patient populations, and rising incidences of cardiac diseases are key drivers of growth in this region.North America Paclitaxeleluting Stent Market Report:
The North American region is prominent in the Paclitaxeleluting Stent market, with a valuation of $0.88 billion in 2023 and a projected $1.74 billion by 2033. High patient awareness, advanced medical research, and technological innovations drive the region's substantial market share.South America Paclitaxeleluting Stent Market Report:
The South American market is relatively nascent, estimated at $0.08 billion in 2023, expected to reach $0.16 billion by 2033. Factors contributing to growth include increasing healthcare investments and improvements in medical facilities.Middle East & Africa Paclitaxeleluting Stent Market Report:
The Middle East and Africa market, accounting for $0.26 billion in 2023, is projected to reach $0.51 billion by 2033. Increasing urbanization, improving healthcare infrastructure, and growing prevalence of heart diseases characterize this market's growth.Tell us your focus area and get a customized research report.
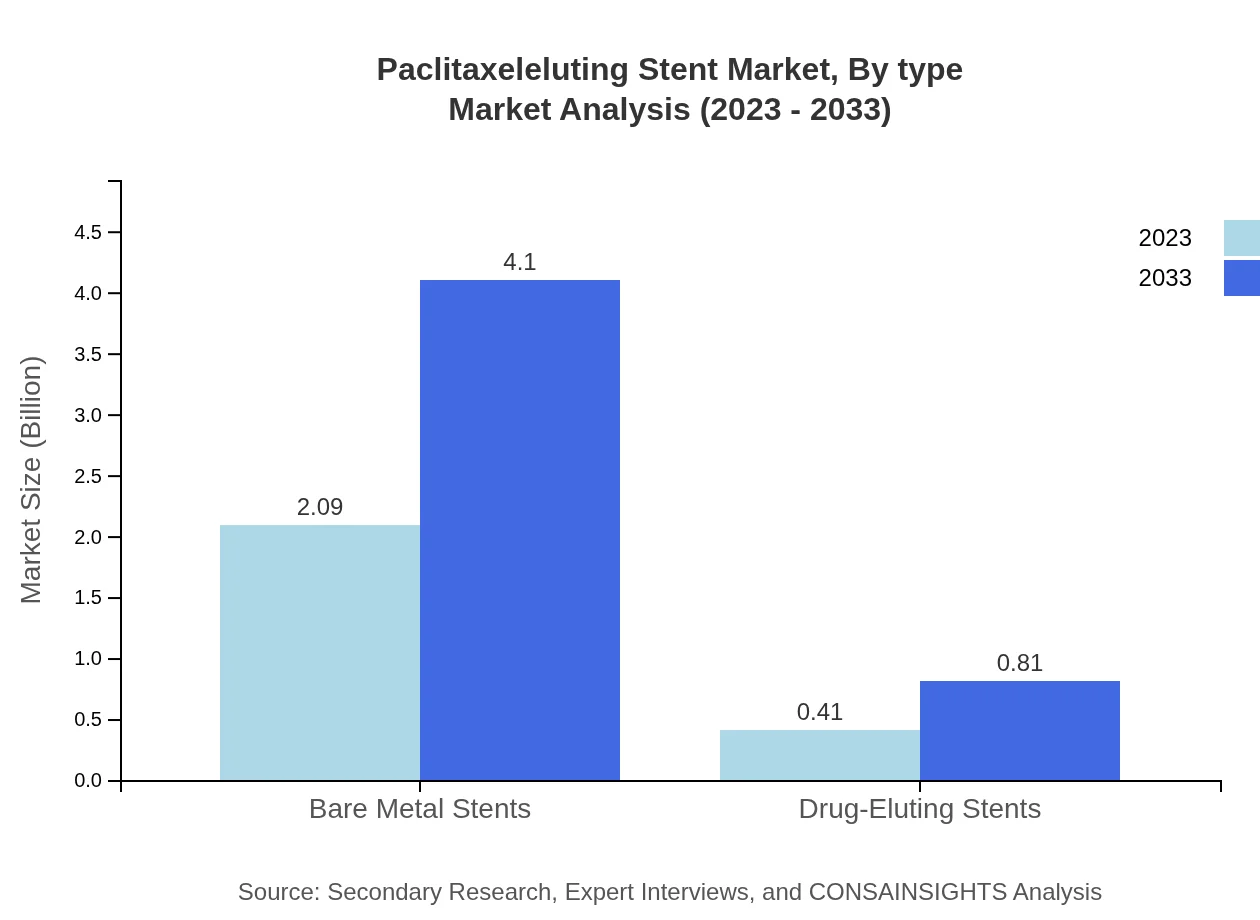
Paclitaxeleluting Stent Market Analysis By Type
The market is largely dominated by Bare Metal Stents, valued at $2.09 billion in 2023, expected to reach $4.10 billion by 2033, holding a share of 83.42%. Drug-Eluting Stents are also significant with a size of $0.41 billion in 2023, projected to reach $0.81 billion by 2033, sharing 16.58%.
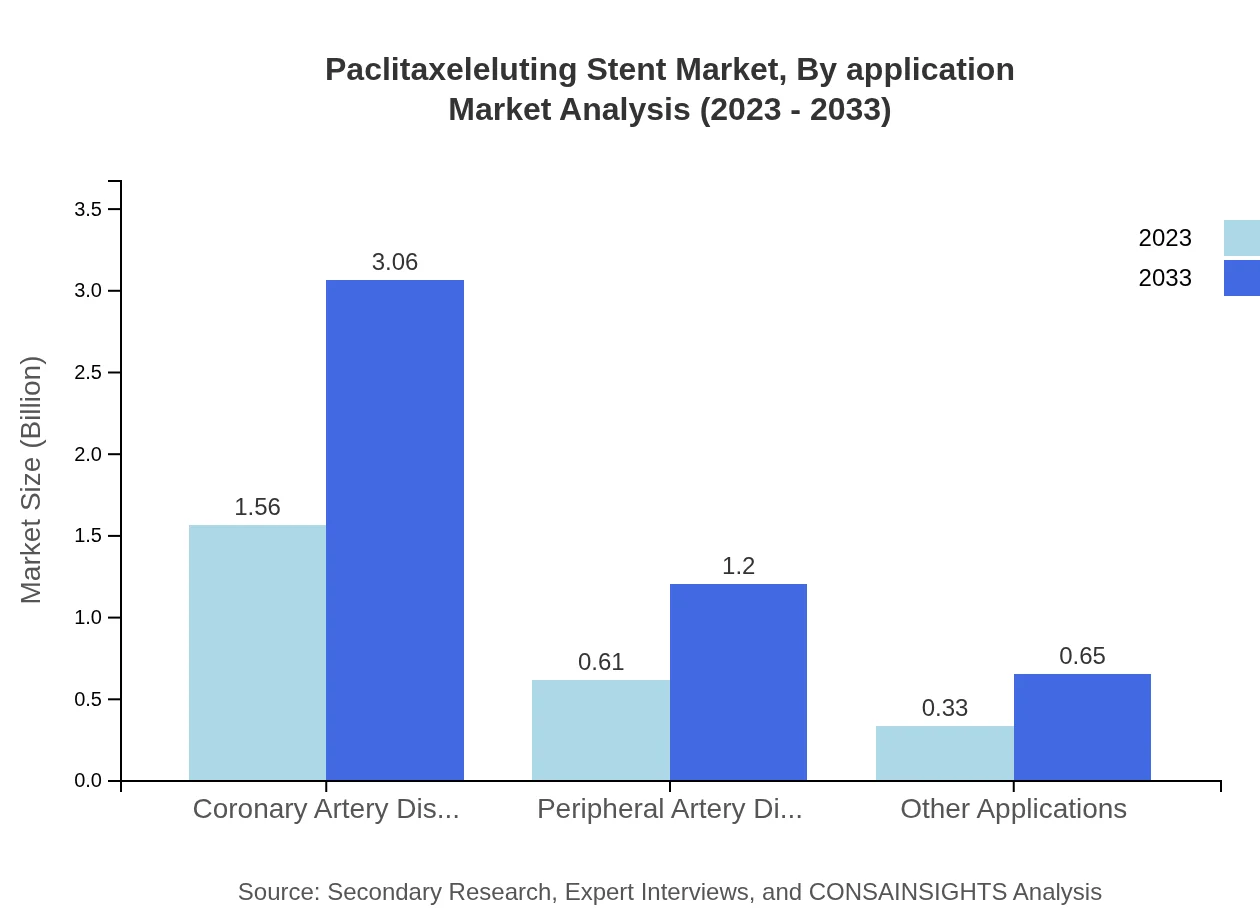
Paclitaxeleluting Stent Market Analysis By Application
The Coronary Artery Disease segment is the largest, valued at $1.56 billion in 2023, expected to grow to $3.06 billion by 2033, representing 62.35%. The Peripheral Artery Disease segment follows closely, valued at $0.61 billion in 2023, anticipated to grow to $1.20 billion by 2033, with a share of 24.34%.
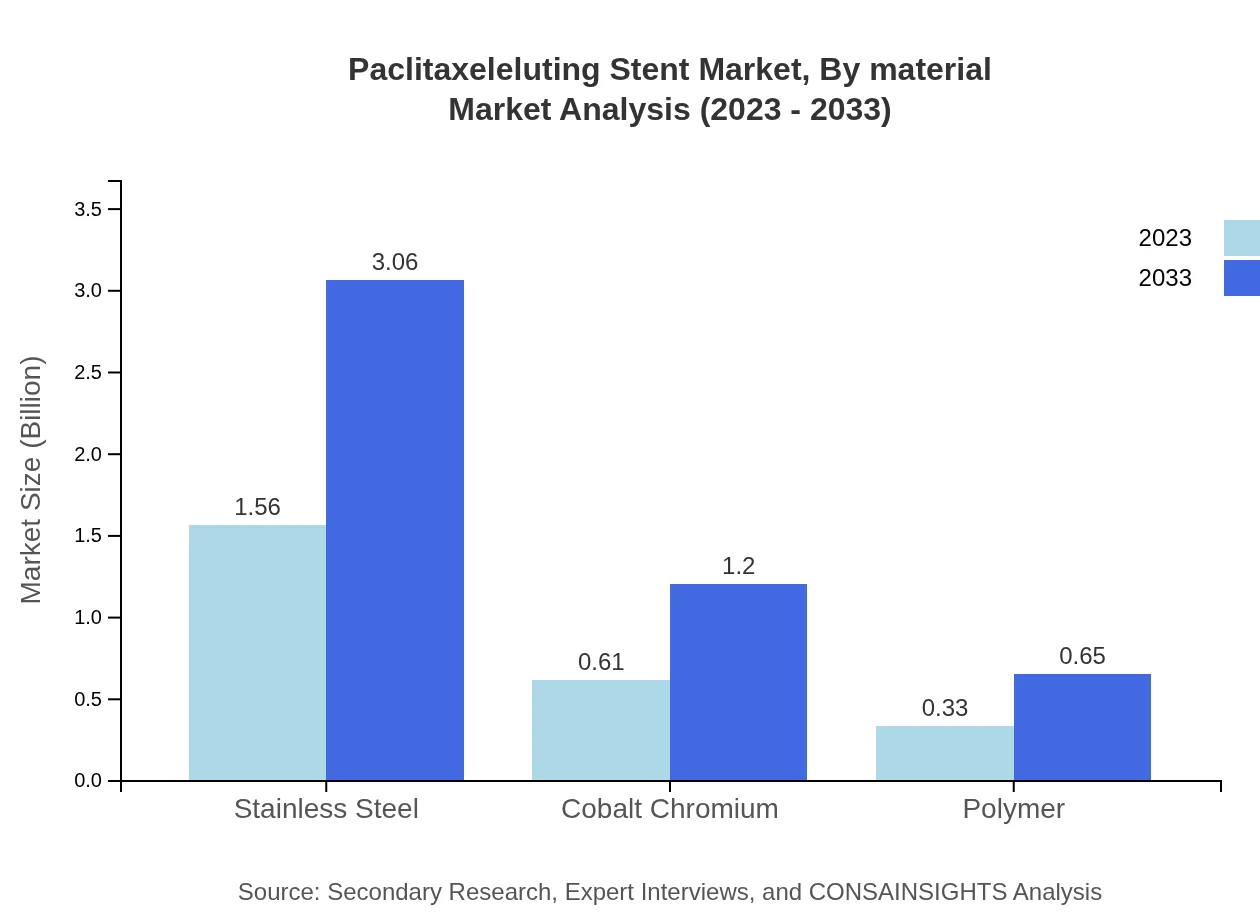
Paclitaxeleluting Stent Market Analysis By Material
The Stainless Steel segment takes a leading position, valued at $1.56 billion in 2023, projecting to $3.06 billion by 2033 (62.35%). The Cobalt Chromium segment, valued at $0.61 billion in 2023, is expected to rise to $1.20 billion within the same timeframe, contributing 24.34%.
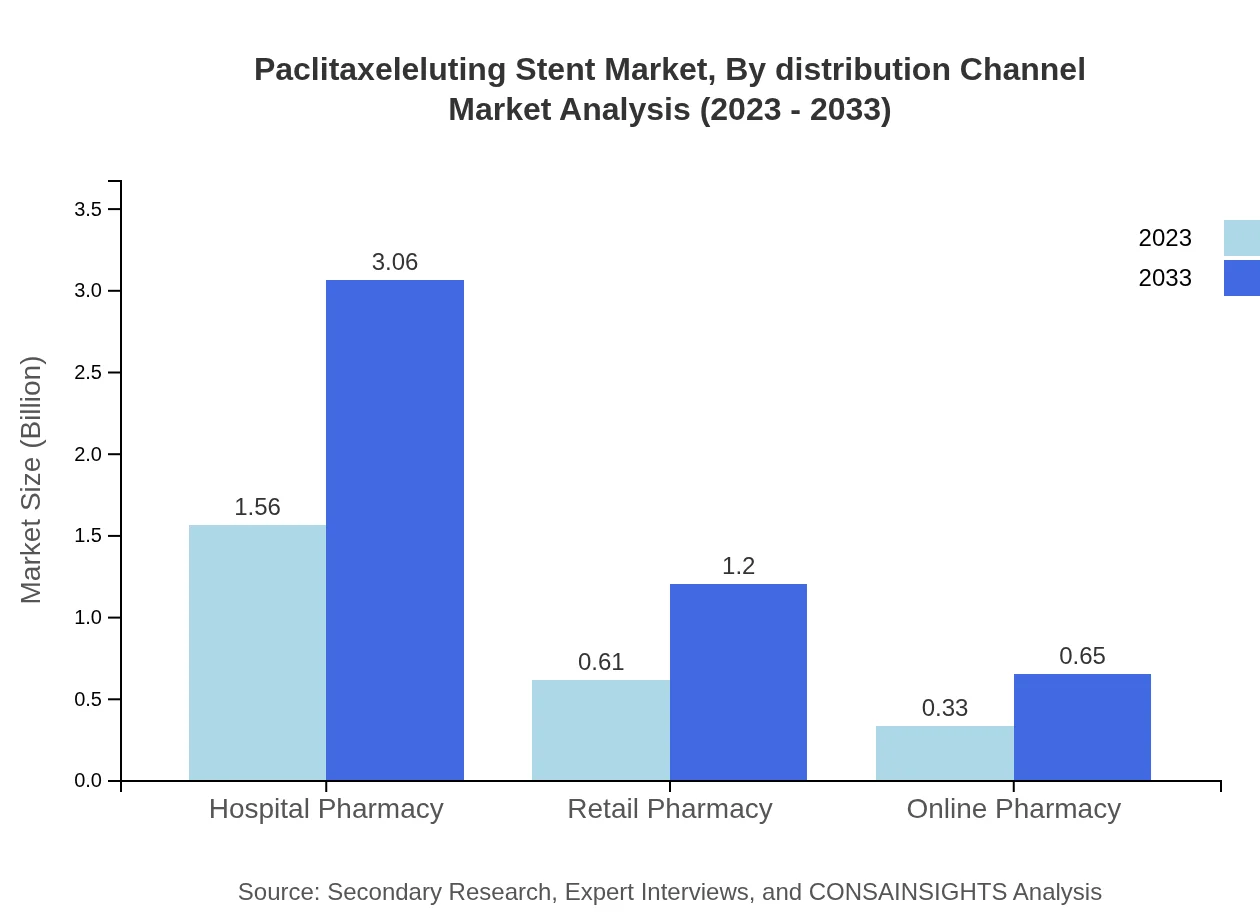
Paclitaxeleluting Stent Market Analysis By Distribution Channel
Hospital Pharmacies dominate the distribution channel, valued at $1.56 billion in 2023, with an anticipated increase to $3.06 billion by 2033. Retail pharmacies and Online channels present growth opportunities but still constitute smaller shares compared to hospital pharmacies.
Paclitaxeleluting Stent Market Trends and Future Forecast
Tell us your focus area and get a customized research report.
Global Market Leaders and Top Companies in Paclitaxeleluting Stent Industry
Medtronic :
A global leader in medical technology, Medtronic offers innovative cardiovascular devices, including advanced drug-eluting stents.Boston Scientific:
Boston Scientific is known for its pioneering medical advancements, producing a range of stents that enhance patient outcomes in cardiovascular treatments.Abbott Laboratories:
Abbott Laboratories focuses on cardiovascular health, equipped with multi-year experience in developing and marketing stents, particularly drug-eluting varieties.B. Braun Melsungen AG:
B. Braun is recognized for its innovative healthcare solutions, including drug-eluting stents, significantly contributing to the market.We're grateful to work with incredible clients.









FAQs
What is the market size of paclitaxeleluting Stent?
The global paclitaxel-eluting stent market is projected to grow from approximately $2.5 billion in 2023 to an estimated size indicating significant expansion. This growth trajectory reflects a compound annual growth rate (CAGR) of 6.8% over the decade.
What are the key market players or companies in the paclitaxeleluting Stent industry?
The paclitaxel-eluting stent industry is competitive, featuring key players including Abbott Laboratories, Boston Scientific Corporation, and Medtronic. These companies are prominent for their innovations and product offerings, leading advancements in stent technologies globally.
What are the primary factors driving the growth in the paclitaxeleluting Stent industry?
Key drivers of growth in the paclitaxel-eluting stent market include rising incidences of cardiovascular diseases, advancements in stent technology, and increasing healthcare expenditure. Additionally, the ageing population and expanding applications further contribute to market expansion.
Which region is the fastest Growing in the paclitaxeleluting stent?
North America leads as the fastest-growing region for paclitaxel-eluting stents, with a projected market increase from $0.88 billion in 2023 to $1.74 billion by 2033. Europe and the Asia Pacific regions also demonstrate significant growth potential.
Does ConsaInsights provide customized market report data for the paclitaxeleluting Stent industry?
Yes, Consainsights offers customized market report data tailored to the specific needs of clients in the paclitaxel-eluting stent industry. This allows businesses to gain precise insights relevant to their strategic objectives.
What deliverables can I expect from this paclitaxeleluting Stent market research project?
Clients can expect comprehensive market analysis reports, detailed segmentation data, growth forecasts, competitive landscape assessments, and region-specific insights tailored to the paclitaxel-eluting stent market.
What are the market trends of paclitaxeleluting Stent?
Emerging trends in the paclitaxel-eluting stent market include the adoption of advanced materials, increased focus on minimally invasive procedures, and the integration of digital technologies in patient monitoring. These trends are shaping the future landscape of the industry.