Pediatric Clinical Trials Market Report
Published Date: 31 January 2026 | Report Code: pediatric-clinical-trials
Pediatric Clinical Trials Market Size, Share, Industry Trends and Forecast to 2033
This report provides a comprehensive analysis of the Pediatric Clinical Trials market, covering insights on market trends, size forecasts, segmentation, and key players for the period 2023-2033.
| Metric | Value |
|---|---|
| Study Period | 2023 - 2033 |
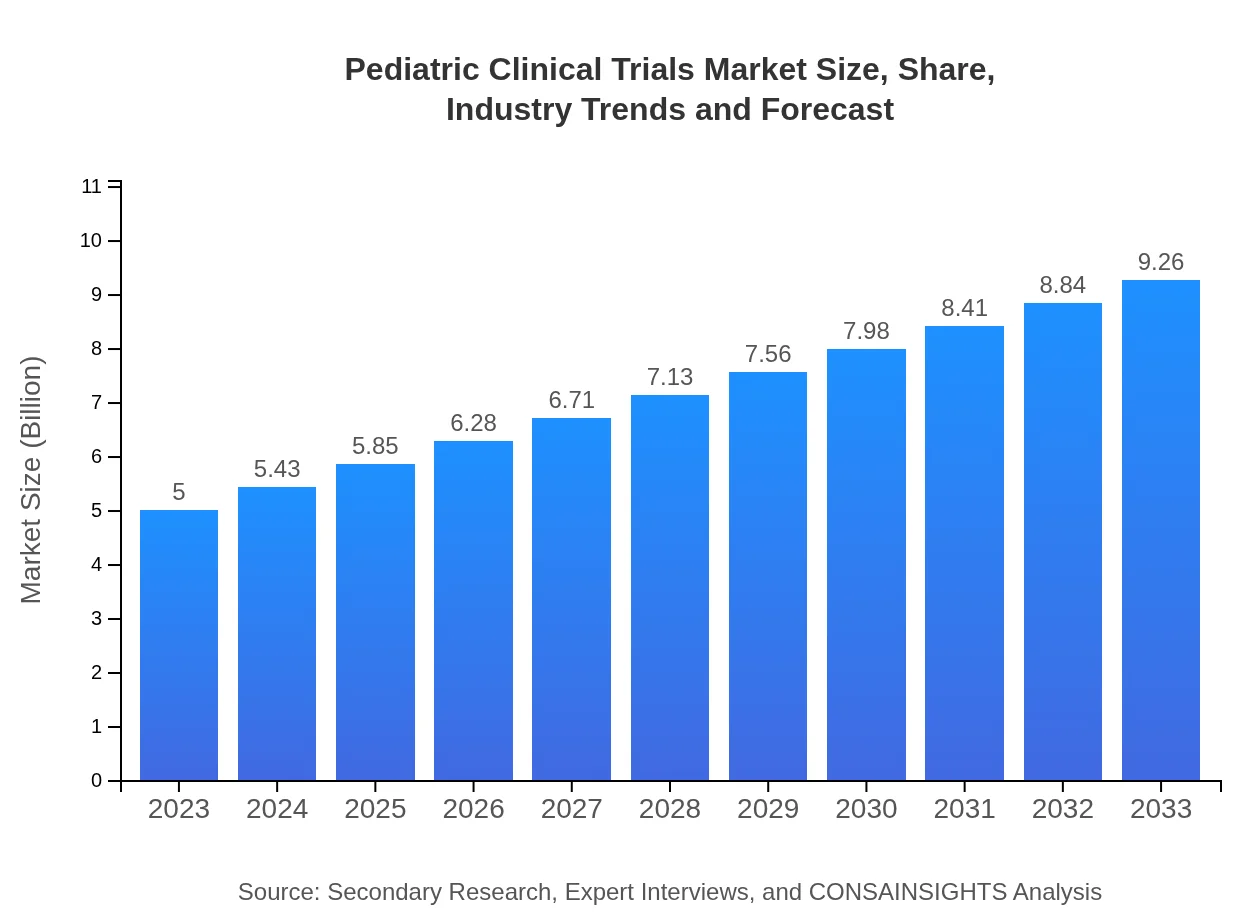
| 2023 Market Size | $5.00 Billion |
| CAGR (2023-2033) | 6.2% |
| 2033 Market Size | $9.26 Billion |
| Top Companies | Pfizer , Roche, Novartis, Johnson & Johnson, Merck & Co. |
| Last Modified Date | 31 January 2026 |
Pediatric Clinical Trials Market Overview
Customize Pediatric Clinical Trials Market Report market research report
- ✔ Get in-depth analysis of Pediatric Clinical Trials market size, growth, and forecasts.
- ✔ Understand Pediatric Clinical Trials's regional dynamics and industry-specific trends.
- ✔ Identify potential applications, end-user demand, and growth segments in Pediatric Clinical Trials
What is the Market Size & CAGR of Pediatric Clinical Trials market in 2023?
Pediatric Clinical Trials Industry Analysis
Pediatric Clinical Trials Market Segmentation and Scope
Tell us your focus area and get a customized research report.
Pediatric Clinical Trials Market Analysis Report by Region
Europe Pediatric Clinical Trials Market Report:
The European market for pediatric clinical trials was valued at $1.72 billion in 2023 and is expected to reach $3.19 billion by 2033. The region's growth is propelled by stringent regulatory requirements encouraging the conducting of pediatric trials alongside adult trials, alongside increased funding in pediatrics.Asia Pacific Pediatric Clinical Trials Market Report:
The Asia Pacific region is expected to witness robust growth in pediatric clinical trials, rising from $0.94 billion in 2023 to $1.73 billion by 2033. This growth can be attributed to improving healthcare infrastructure, increasing awareness of pediatric healthcare, and government incentives aimed at bolstering clinical research.North America Pediatric Clinical Trials Market Report:
North America leads the market with a valuation of $1.70 billion in 2023, projected to climb to $3.15 billion by 2033. Factors driving this growth include an established framework for conducting trials, significant public and private funding, and a growing focus on pediatric health issues in research agendas.South America Pediatric Clinical Trials Market Report:
South America's market, starting at $0.36 billion in 2023, is anticipated to grow to $0.67 billion by 2033. This region faces challenges such as economic instability and regulatory hurdles but benefits from increased international collaboration and funding for pediatric studies.Middle East & Africa Pediatric Clinical Trials Market Report:
The Middle East and Africa's market, starting at $0.29 billion in 2023 and projected to reach $0.53 billion by 2033, is influenced by growing healthcare needs, emerging trial designs, and increasing multi-center trial collaborations enhancing research capabilities.Tell us your focus area and get a customized research report.
Pediatric Clinical Trials Market Analysis By Study Phase
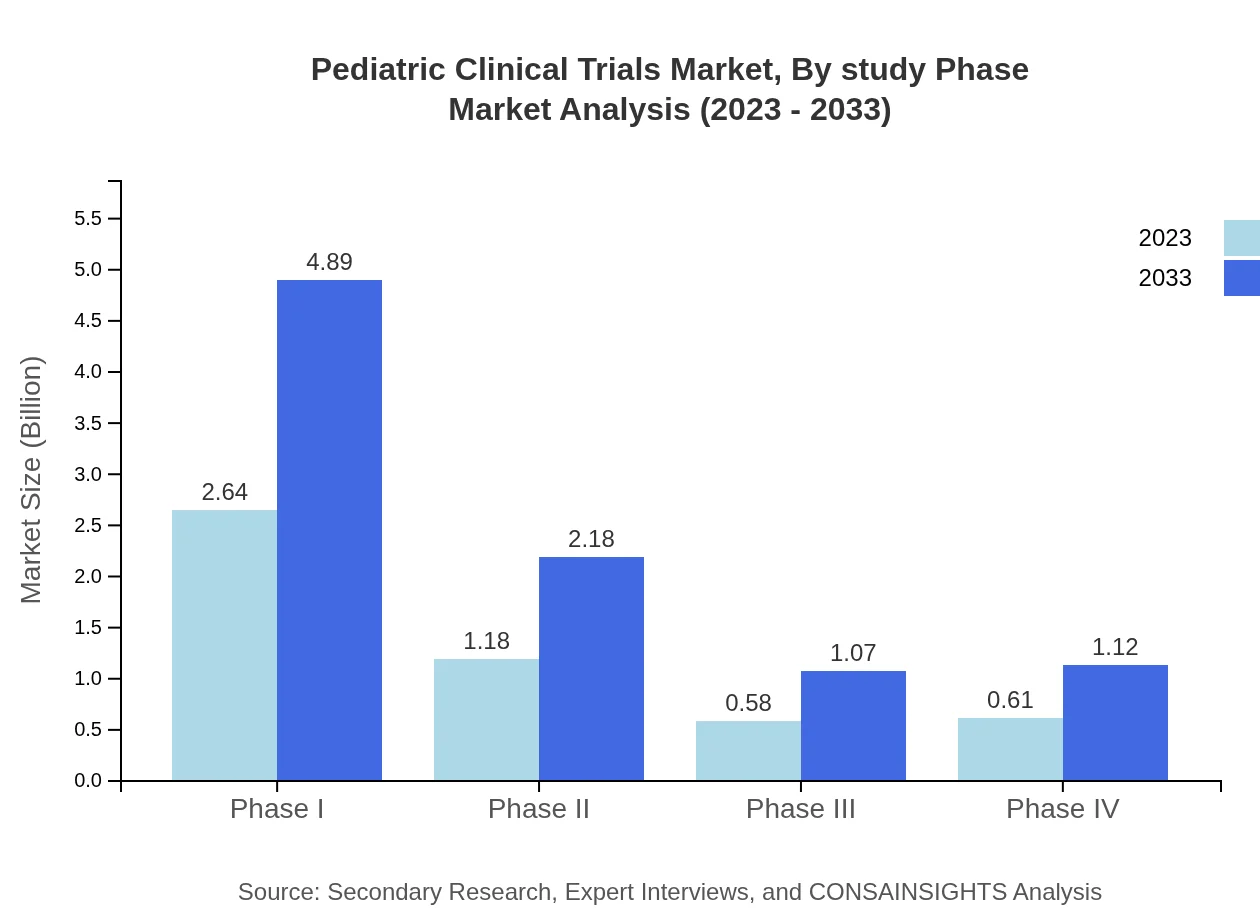
The Pediatric Clinical Trials market is significantly influenced by its study phases. In 2023, Phase I trials represented a market size of $2.64 billion and are expected to grow to $4.89 billion by 2033. Approximately 52.83% of the market share stems from this phase, given its vital role in assessing safety. Phase II trials contribute a size of $1.18 billion with a market share of 23.51%, moving to $2.18 billion by 2033. Phase III trials account for $0.58 billion in 2023 and are projected to reach $1.07 billion, maintaining an 11.54% share, while Phase IV trials will grow from $0.61 billion to $1.12 billion with a 12.12% share.
Pediatric Clinical Trials Market Analysis By Indication
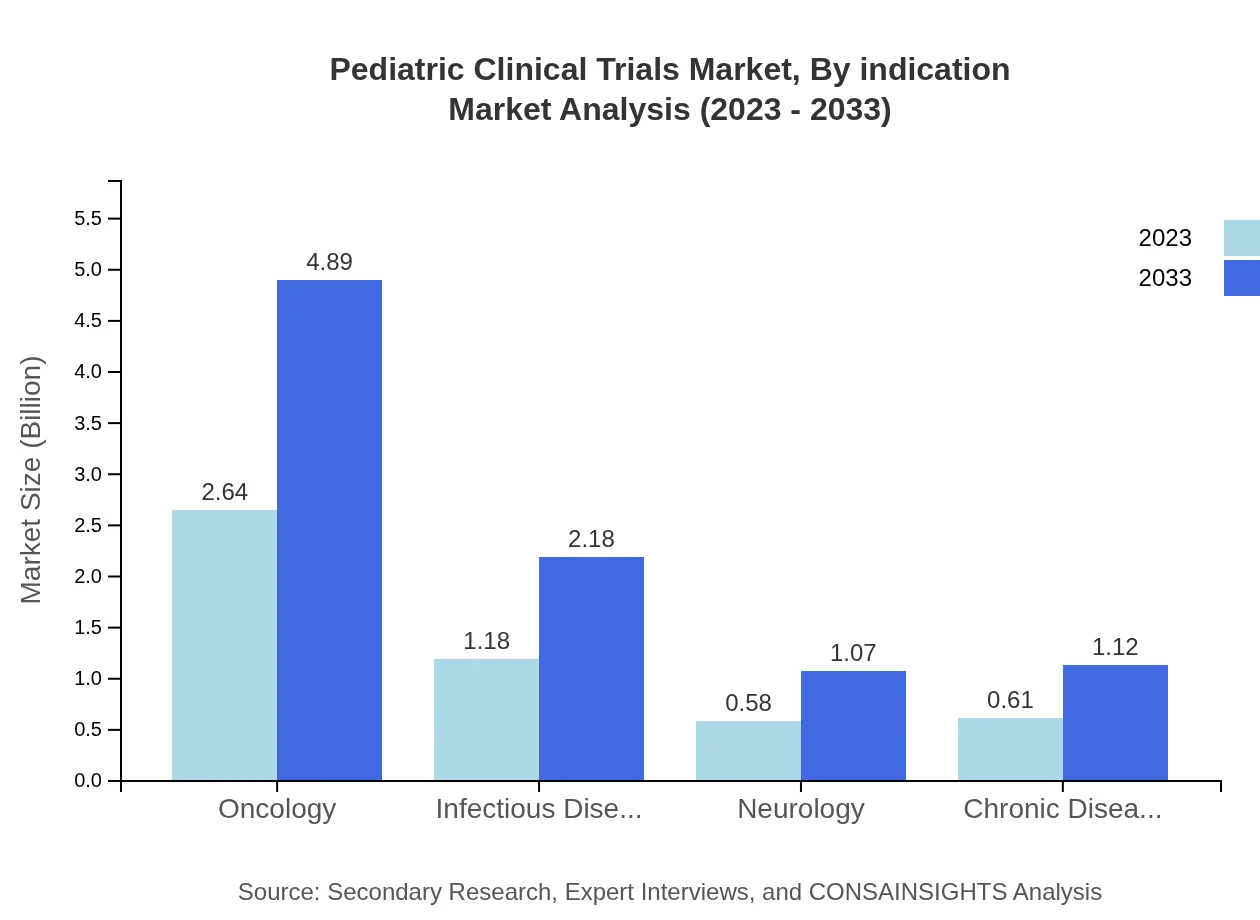
When analyzing by indication, oncology is a primary focus, with a market size of $2.64 billion in 2023, expected to reach $4.89 billion by 2033, representing 52.83% of the market. Infectious diseases follow with a market size of $1.18 billion and 23.51% share, growing to $2.18 billion. Neurology represents a smaller proportion with $0.58 billion projected to rise to $1.07 billion. Chronic diseases contribute significantly as well, with an expected rise from $0.61 billion to $1.12 billion.
Pediatric Clinical Trials Market Analysis By Design
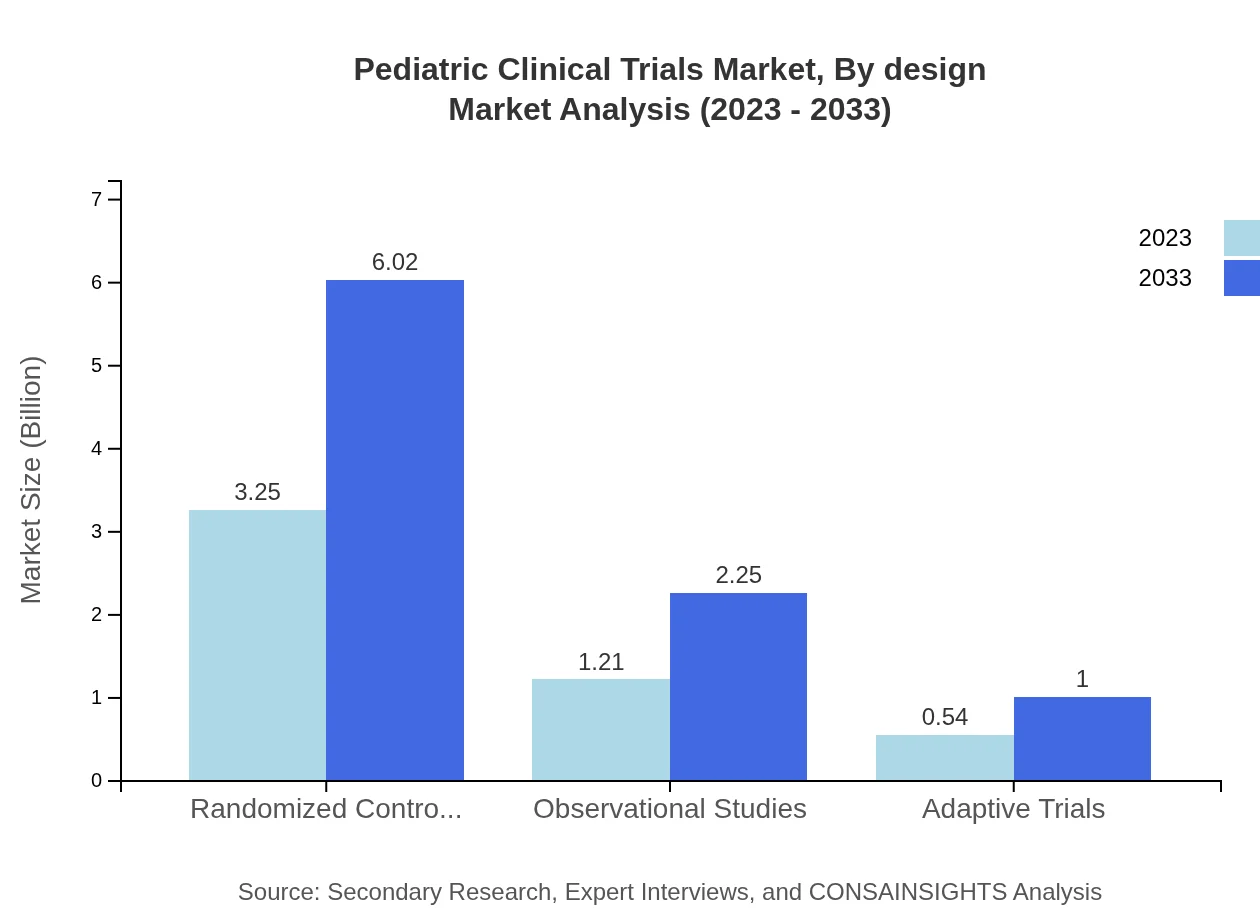
The trials can be categorized by design into randomized controlled trials, observational studies, and adaptive trials. In 2023, randomized controlled trials dominated the market, with a size of $3.25 billion and 64.94% market share, expected to rise to $6.02 billion. Observational studies encompass $1.21 billion in 2023 with a 24.24% share, growing to $2.25 billion. Adaptive trials account for a smaller segment, starting at $0.54 billion and projected to reach $1.00 billion.
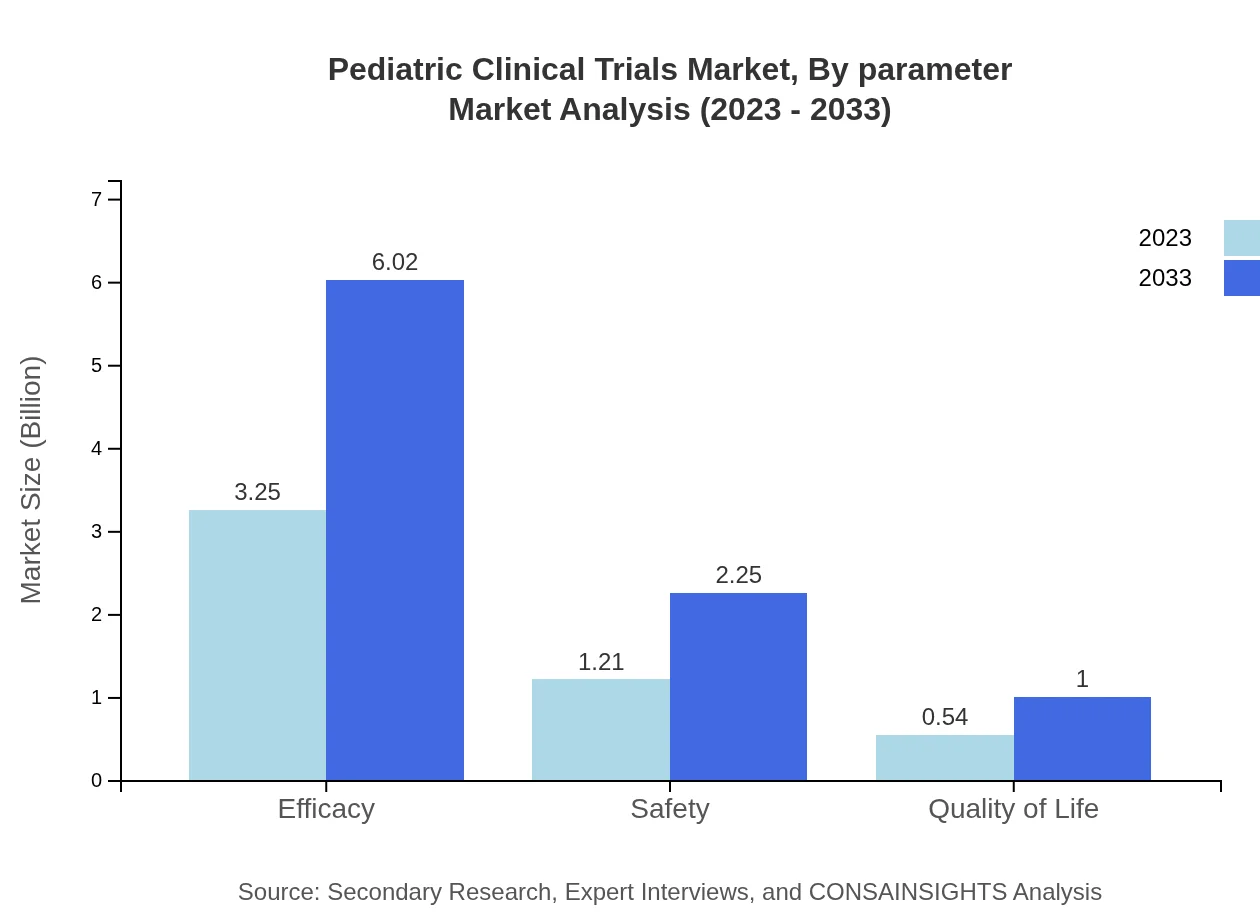
Pediatric Clinical Trials Market Analysis By Parameter
In terms of market parameters for evaluation, efficacy and safety remain leading aspects. Efficacy trials are expected to generate a substantial portion of revenue, starting at $3.25 billion with 64.94% share, rising to $6.02 billion. Safety trials follow, beginning at $1.21 billion, moving to $2.25 billion, while quality of life evaluations are also significant, ascending from $0.54 billion to $1.00 billion throughout this period.
Pediatric Clinical Trials Market Trends and Future Forecast
Tell us your focus area and get a customized research report.
Global Market Leaders and Top Companies in Pediatric Clinical Trials Industry
Pfizer :
Pfizer is a global biopharmaceutical company that invests heavily in pediatric research, ensuring new therapies cater to children's health needs, particularly in oncology and infectious diseases.Roche:
Roche leads in developing innovative therapies for pediatric populations, focusing on precision medicine and has established strong partnerships with regulatory bodies to enhance pediatric trial efficiency.Novartis:
Novartis is committed to addressing unmet medical needs in children through extensive research and development efforts, focusing on various therapeutic areas, including rare diseases and neurological disorders.Johnson & Johnson:
Johnson & Johnson is known for its comprehensive portfolio of pediatric studies, emphasizing safety and efficacy, and contributing significantly to developing therapies for various pediatric indications.Merck & Co.:
Merck is actively involved in pediatric clinical trials, with a focus on developing vaccines and therapeutic approaches in infectious diseases, enhancing children’s health globally.We're grateful to work with incredible clients.









FAQs
What is the market size of pediatric Clinical Trials?
The pediatric clinical trials market is currently valued at approximately $5 billion in 2023 and is projected to grow at a CAGR of 6.2% through 2033, reflecting increasing demand for child-focused medical research.
What are the key market players or companies in this pediatric Clinical Trials industry?
Key players in the pediatric clinical trials industry include pharmaceutical companies focusing on pediatric formulations, contract research organizations (CROs), and academic institutions conducting research, ensuring comprehensive engagement in this specialized market.
What are the primary factors driving the growth in the pediatric Clinical Trials industry?
The growth in the pediatric clinical trials industry is driven by factors such as rising pediatric disease prevalence, increased regulatory focus on child safety in clinical research, and enhanced technological developments aimed at improving trial efficiency and data accuracy.
Which region is the fastest Growing in the pediatric Clinical Trials?
The fastest-growing region in the pediatric clinical trials market is expected to be Asia Pacific, projected to grow from $0.94 billion in 2023 to $1.73 billion by 2033, fueled by increasing healthcare investments and improved clinical research infrastructure.
Does ConsaInsights provide customized market report data for the pediatric Clinical Trials industry?
Yes, ConsaInsights offers customized market report data tailored to meet specific needs in the pediatric clinical trials industry, ensuring clients receive relevant insights for strategic decision-making and market positioning.
What deliverables can I expect from this pediatric Clinical Trials market research project?
Deliverables from a pediatric clinical trials market research project include comprehensive market analysis reports, detailed segmentation data by type and phase, competitive landscape evaluations, and tailored recommendations based on market trends.
What are the market trends of pediatric Clinical Trials?
Key trends in the pediatric clinical trials market include an increasing shift towards adaptive trial designs, greater emphasis on patient-centric approaches, and rising collaboration between industry stakeholders to enhance trial feasibility and outcome reliability.