Pediatric Drugs Market Report
Published Date: 31 January 2026 | Report Code: pediatric-drugs
Pediatric Drugs Market Size, Share, Industry Trends and Forecast to 2033
This comprehensive report explores the Pediatric Drugs market, providing insights on market size, growth rates, regional analysis, and future forecasts from 2023 to 2033.
| Metric | Value |
|---|---|
| Study Period | 2023 - 2033 |
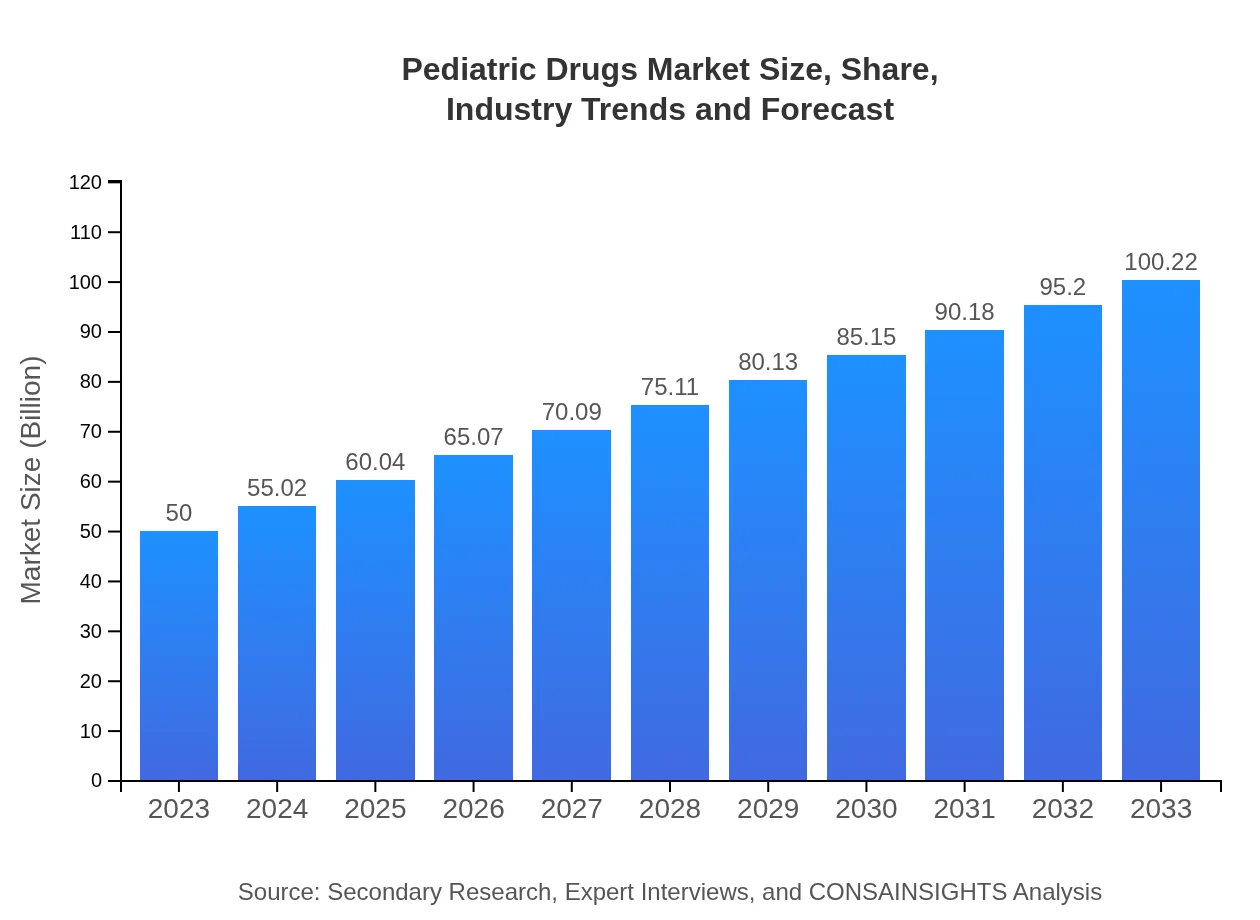
| 2023 Market Size | $50.00 Billion |
| CAGR (2023-2033) | 7% |
| 2033 Market Size | $100.22 Billion |
| Top Companies | Pfizer Inc., Roche, GlaxoSmithKline, Novartis, Bristol-Myers Squibb |
| Last Modified Date | 31 January 2026 |
Pediatric Drugs Market Overview
Customize Pediatric Drugs Market Report market research report
- ✔ Get in-depth analysis of Pediatric Drugs market size, growth, and forecasts.
- ✔ Understand Pediatric Drugs's regional dynamics and industry-specific trends.
- ✔ Identify potential applications, end-user demand, and growth segments in Pediatric Drugs
What is the Market Size & CAGR of Pediatric Drugs market in 2023?
Pediatric Drugs Industry Analysis
Pediatric Drugs Market Segmentation and Scope
Tell us your focus area and get a customized research report.
Pediatric Drugs Market Analysis Report by Region
Europe Pediatric Drugs Market Report:
The European Pediatric Drugs market is forecasted to grow from $14.18 billion in 2023 to $28.42 billion by 2033. The commitment from regulatory authorities towards developing pediatric-specific formulations and increasing awareness about the need for child-safe medications are key growth drivers.Asia Pacific Pediatric Drugs Market Report:
In the Asia Pacific region, the Pediatric Drugs market is expected to increase from $9.41 billion in 2023 to $18.86 billion by 2033. The growth is driven by rising healthcare expenditures and increasing child population. Advancements in healthcare infrastructure and government initiatives to improve pediatric healthcare further support market expansion.North America Pediatric Drugs Market Report:
North America remains a major market for Pediatric Drugs, anticipated to rise from $18.93 billion in 2023 to $37.94 billion by 2033. The region benefits from a high prevalence of childhood diseases, strong regulatory frameworks, and robust R&D activities targeting pediatric drugs.South America Pediatric Drugs Market Report:
The Pediatric Drugs market in South America is projected to grow from $1.87 billion in 2023 to $3.75 billion by 2033. Factors influencing this growth include improving access to healthcare services, rising awareness of child health, and the introduction of affordable pediatric medication.Middle East & Africa Pediatric Drugs Market Report:
In the Middle East and Africa, the market is expected to increase from $5.61 billion in 2023 to $11.24 billion by 2033. Growth is propelled by improving healthcare systems, increasing child population, and rising investment in pediatric healthcare initiatives.Tell us your focus area and get a customized research report.
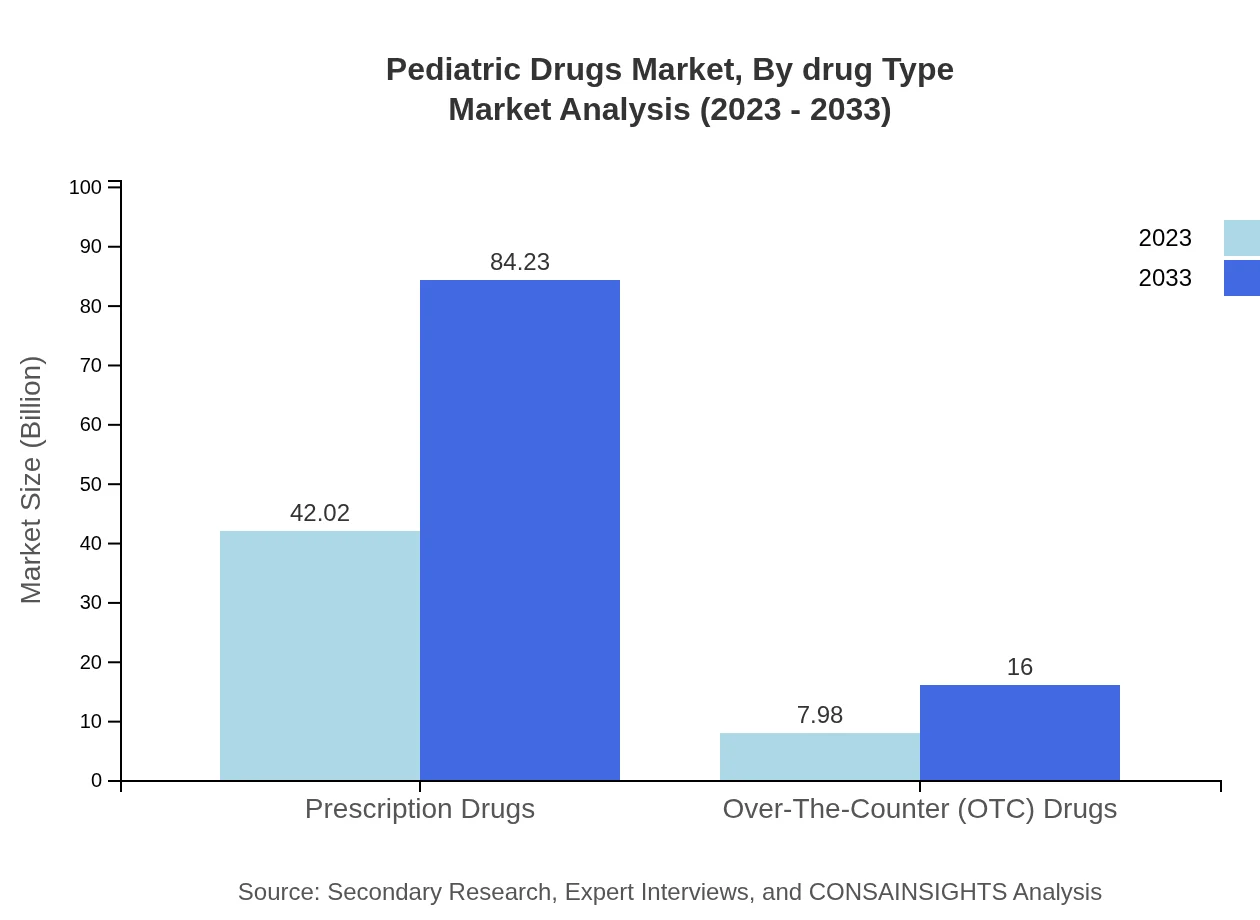
Pediatric Drugs Market Analysis By Drug Type
The Pediatric Drugs market is segmented into prescription and over-the-counter (OTC) drugs. In 2023, prescription drugs alone contribute approximately $42.02 billion to the market, projected to double to $84.23 billion by 2033. OTC drugs, while smaller in comparison, are vital, generating $7.98 billion in 2023 with projected growth to $16.00 billion by 2033.
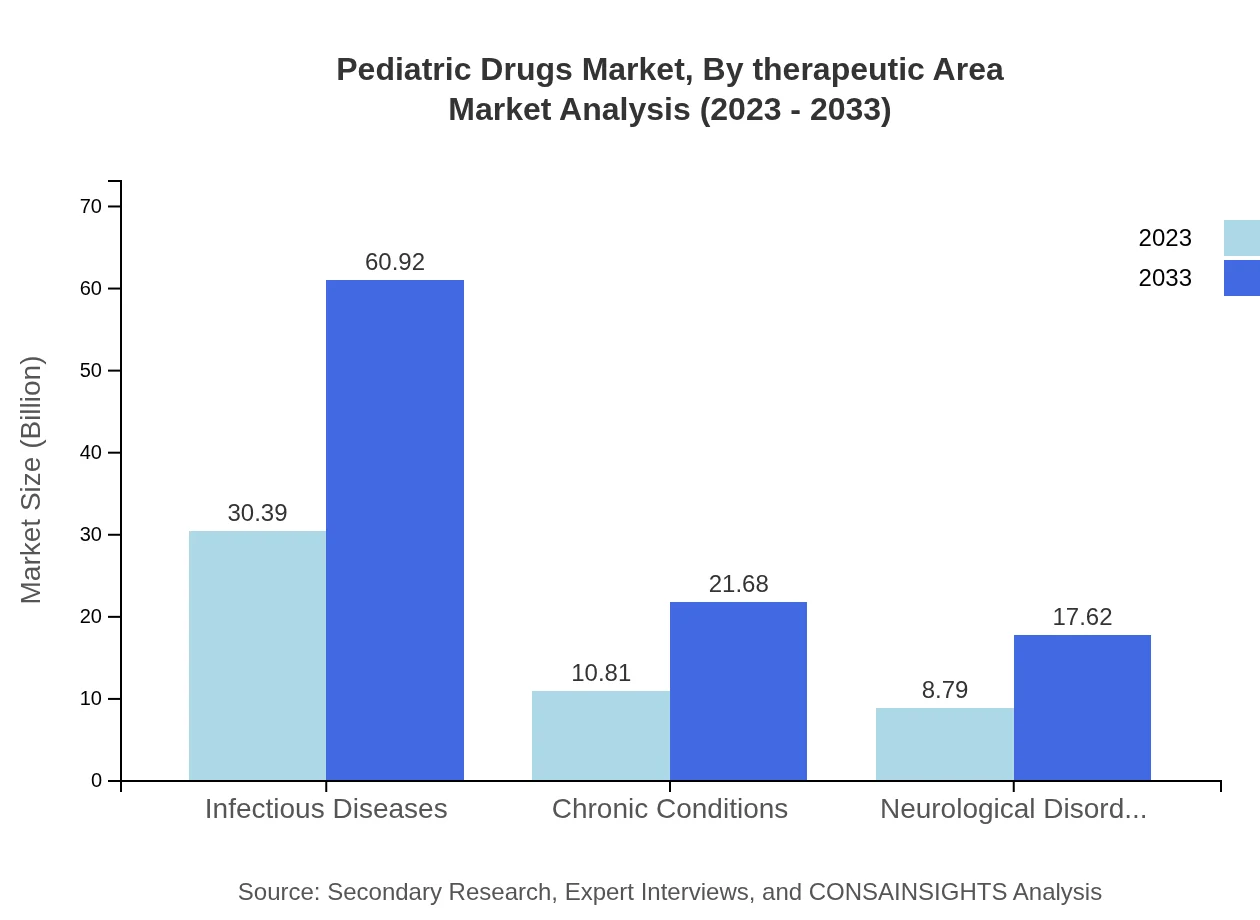
Pediatric Drugs Market Analysis By Therapeutic Area
Therapeutically, the market is primarily segmented into areas such as infectious diseases, chronic conditions, and neurological disorders. The infectious diseases segment dominates, accounting for $30.39 billion in 2023, with an anticipated rise to $60.92 billion by 2033.
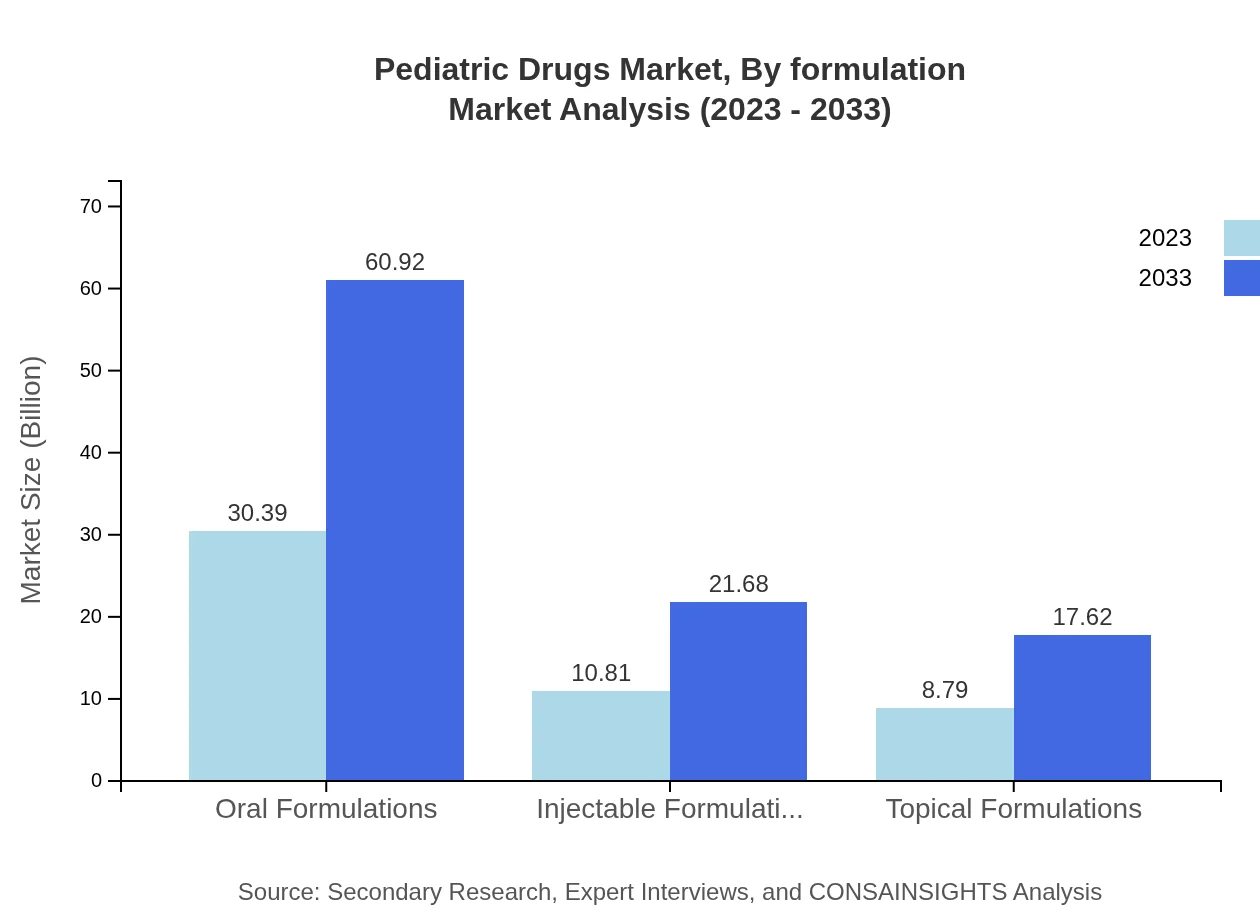
Pediatric Drugs Market Analysis By Formulation
The formulation segment includes oral, injectable, and topical formulations. Oral formulations lead the market with a share of $30.39 billion in 2023 and growing to $60.92 billion by 2033. Injectable formulations, important for severe conditions, are expected to grow from $10.81 billion to $21.68 billion.
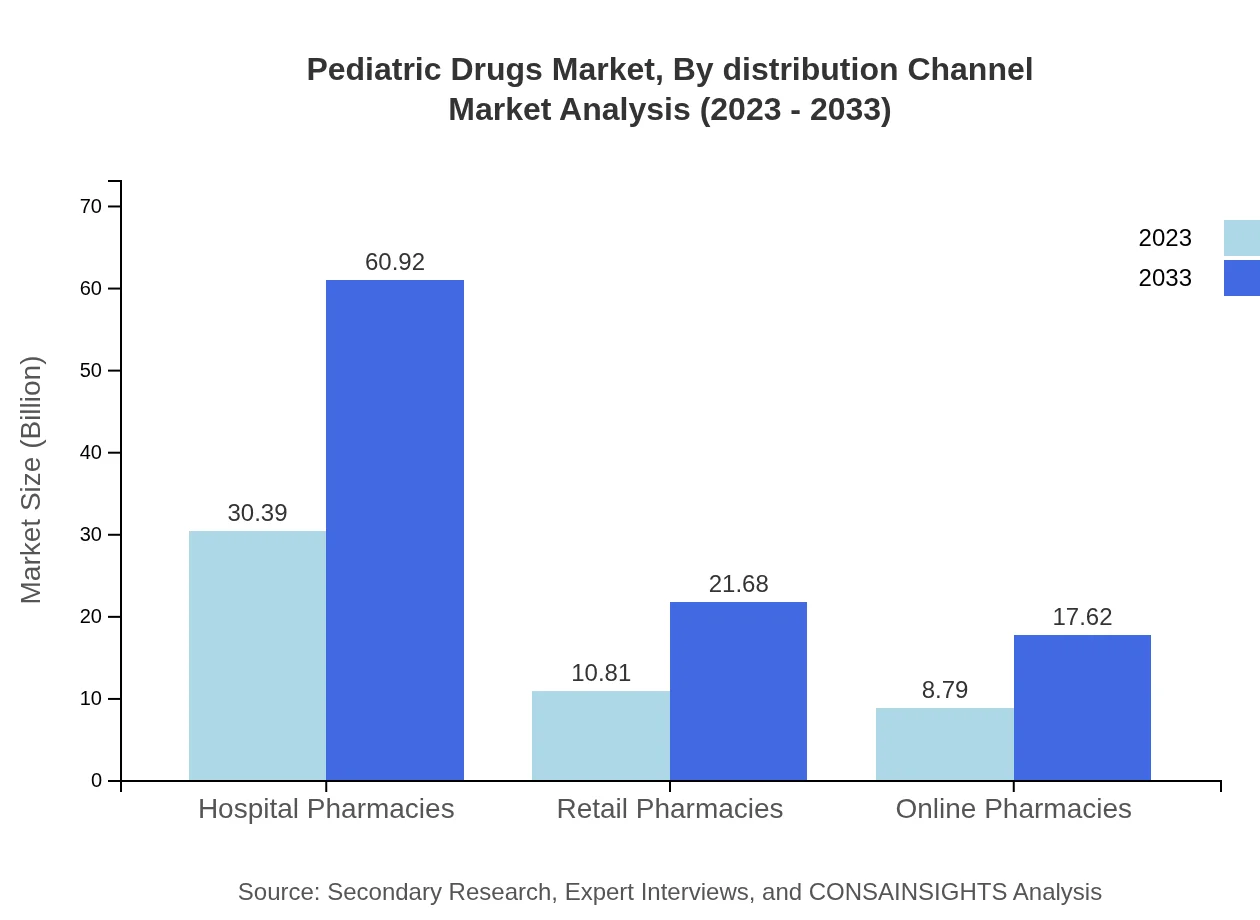
Pediatric Drugs Market Analysis By Distribution Channel
Distribution channels are segmented into hospital pharmacies, retail pharmacies, and online pharmacies. Hospital pharmacies account for a significant market share, expected to rise from $30.39 billion in 2023 to $60.92 billion by 2033, showcasing the importance of hospitals in dispensing pediatric drugs.
Pediatric Drugs Market Trends and Future Forecast
Tell us your focus area and get a customized research report.
Global Market Leaders and Top Companies in Pediatric Drugs Industry
Pfizer Inc.:
Pfizer is a leading global biopharmaceutical company renowned for its diverse range of pediatric medicines, focusing on safety and efficacy tailored for children.Roche:
Roche specializes in innovative treatments and is dedicated to advancing pediatric health with numerous pediatric-specific formulations within their portfolio.GlaxoSmithKline:
GlaxoSmithKline is committed to developing and marketing effective pediatric medications, leveraging extensive research in child health.Novartis:
Novartis is recognized for its contributions to pediatric therapeutics, focusing on accessibility and improvement of health outcomes in children.Bristol-Myers Squibb:
Bristol-Myers Squibb actively invests in pediatric drug development, working towards innovative therapies that address childhood diseases.We're grateful to work with incredible clients.









FAQs
What is the market size of pediatric Drugs?
The pediatric drugs market is valued at approximately $50 billion in 2023 with a projected CAGR of 7% from 2023 to 2033, indicating significant growth potential in addressing the healthcare needs of children.
What are the key market players or companies in this pediatric Drugs industry?
Key players in the pediatric drugs industry include major pharmaceutical companies like Pfizer, Merck, Novartis, Johnson & Johnson, and Roche, which focus on innovative drug development and expanding their pediatric portfolios.
What are the primary factors driving the growth in the pediatric drugs industry?
Growth in the pediatric drugs market is primarily driven by increasing prevalence of childhood diseases, rising healthcare expenditures, advancements in drug formulations, and a growing focus on tailored therapies for children.
Which region is the fastest Growing in the pediatric Drugs?
The fastest-growing region in the pediatric drugs market is North America, projected to grow from $18.93 billion in 2023 to $37.94 billion by 2033, due to high healthcare spending and robust pharmaceutical infrastructure.
Does ConsaInsights provide customized market report data for the pediatric Drugs industry?
Yes, ConsaInsights specializes in providing customized market report data tailored to the unique needs and specifications of clients in the pediatric drugs industry.
What deliverables can I expect from this pediatric Drugs market research project?
Clients can expect comprehensive market analysis reports including market size, growth forecasts, competitive landscape, segment analysis, regional insights, and key trends impacting the pediatric drugs market.
What are the market trends of pediatric Drugs?
Current trends in the pediatric drugs market include increasing adoption of personalized medicine, a rise in digital health technologies, expanding presence of generic drugs, and a focus on developing innovative drug delivery systems.