Pediatric Neuroblastoma Treatment Market Report
Published Date: 31 January 2026 | Report Code: pediatric-neuroblastoma-treatment
Pediatric Neuroblastoma Treatment Market Size, Share, Industry Trends and Forecast to 2033
This report provides a comprehensive analysis of the Pediatric Neuroblastoma Treatment market, offering insights into market size, growth projections, segmentation, and industry dynamics. The forecast period spans from 2023 to 2033.
| Metric | Value |
|---|---|
| Study Period | 2023 - 2033 |
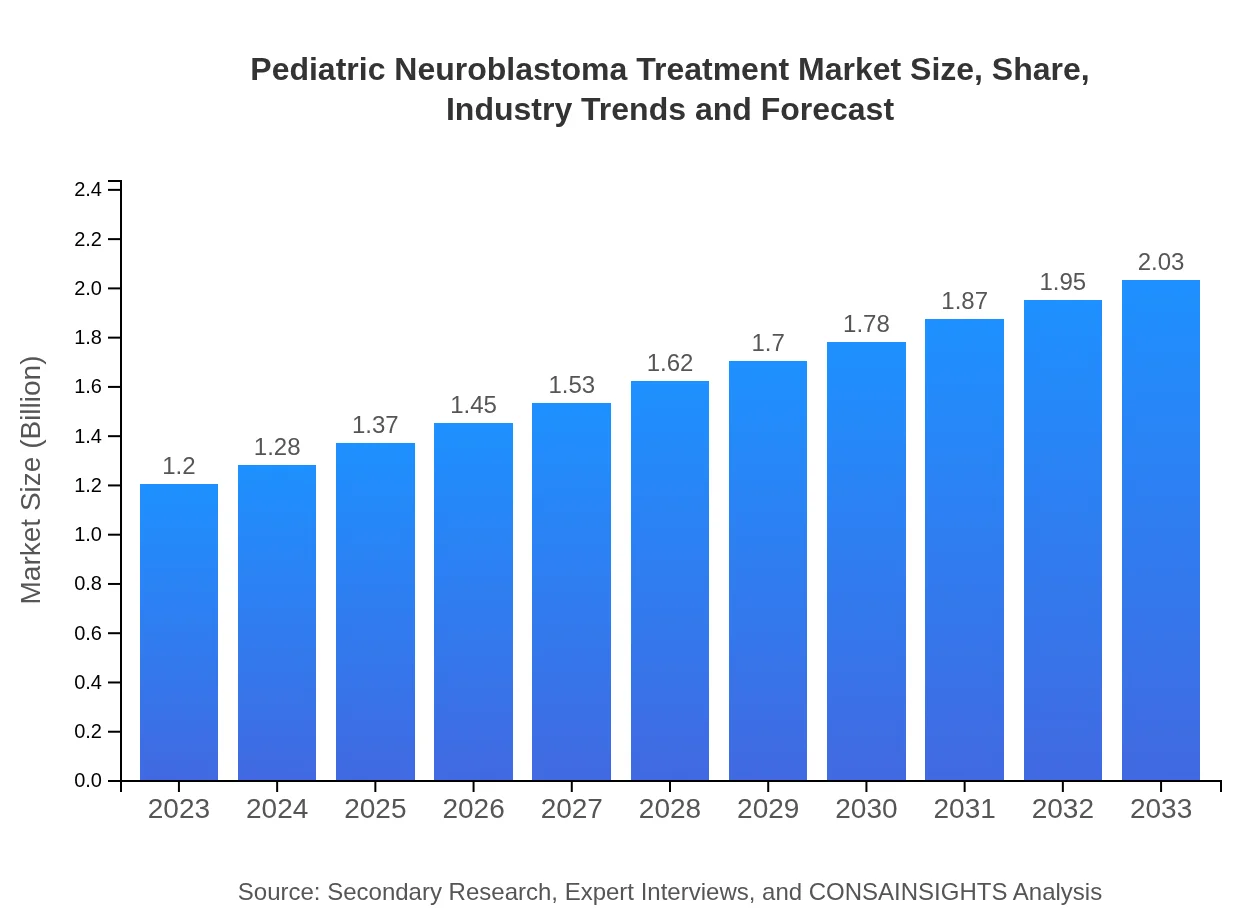
| 2023 Market Size | $1.20 Billion |
| CAGR (2023-2033) | 5.3% |
| 2033 Market Size | $2.03 Billion |
| Top Companies | Novartis AG, Roche Holding AG, Bristol Myers Squibb Co., Eli Lilly and Company, Sanofi |
| Last Modified Date | 31 January 2026 |
Pediatric Neuroblastoma Treatment Market Overview
Customize Pediatric Neuroblastoma Treatment Market Report market research report
- ✔ Get in-depth analysis of Pediatric Neuroblastoma Treatment market size, growth, and forecasts.
- ✔ Understand Pediatric Neuroblastoma Treatment's regional dynamics and industry-specific trends.
- ✔ Identify potential applications, end-user demand, and growth segments in Pediatric Neuroblastoma Treatment
What is the Market Size & CAGR of Pediatric Neuroblastoma Treatment market in 2023?
Pediatric Neuroblastoma Treatment Industry Analysis
Pediatric Neuroblastoma Treatment Market Segmentation and Scope
Tell us your focus area and get a customized research report.
Pediatric Neuroblastoma Treatment Market Analysis Report by Region
Europe Pediatric Neuroblastoma Treatment Market Report:
The European market stands at 0.37 billion USD in 2023 and is expected to grow to 0.62 billion USD by 2033. The growth is supported by stringent regulations ensuring safe and effective treatment practices coupled with high patient awareness.Asia Pacific Pediatric Neuroblastoma Treatment Market Report:
In 2023, the Pediatric Neuroblastoma Treatment market in the Asia Pacific region is valued at 0.23 billion USD and is expected to grow to 0.39 billion USD by 2033. This growth is attributed to improving healthcare infrastructure and increasing awareness about pediatric cancers.North America Pediatric Neuroblastoma Treatment Market Report:
North America dominates the market with a size of 0.43 billion USD in 2023, projected to reach 0.72 billion USD by 2033. This growth is driven by the presence of advanced healthcare facilities, a robust pipeline of drug approvals, and increasing funding for pediatric oncology research.South America Pediatric Neuroblastoma Treatment Market Report:
The South American market is valued at 0.11 billion USD in 2023, with an anticipated rise to 0.19 billion USD by 2033. Factors contributing to this growth include rising healthcare expenditure and government initiatives for childhood cancer treatments.Middle East & Africa Pediatric Neuroblastoma Treatment Market Report:
The market in the Middle East and Africa is valued at 0.07 billion USD in 2023, growing to 0.11 billion USD by 2033. Limited access to advanced healthcare, alongside increasing support for international collaborations in pediatric treatment options, are driving market growth.Tell us your focus area and get a customized research report.
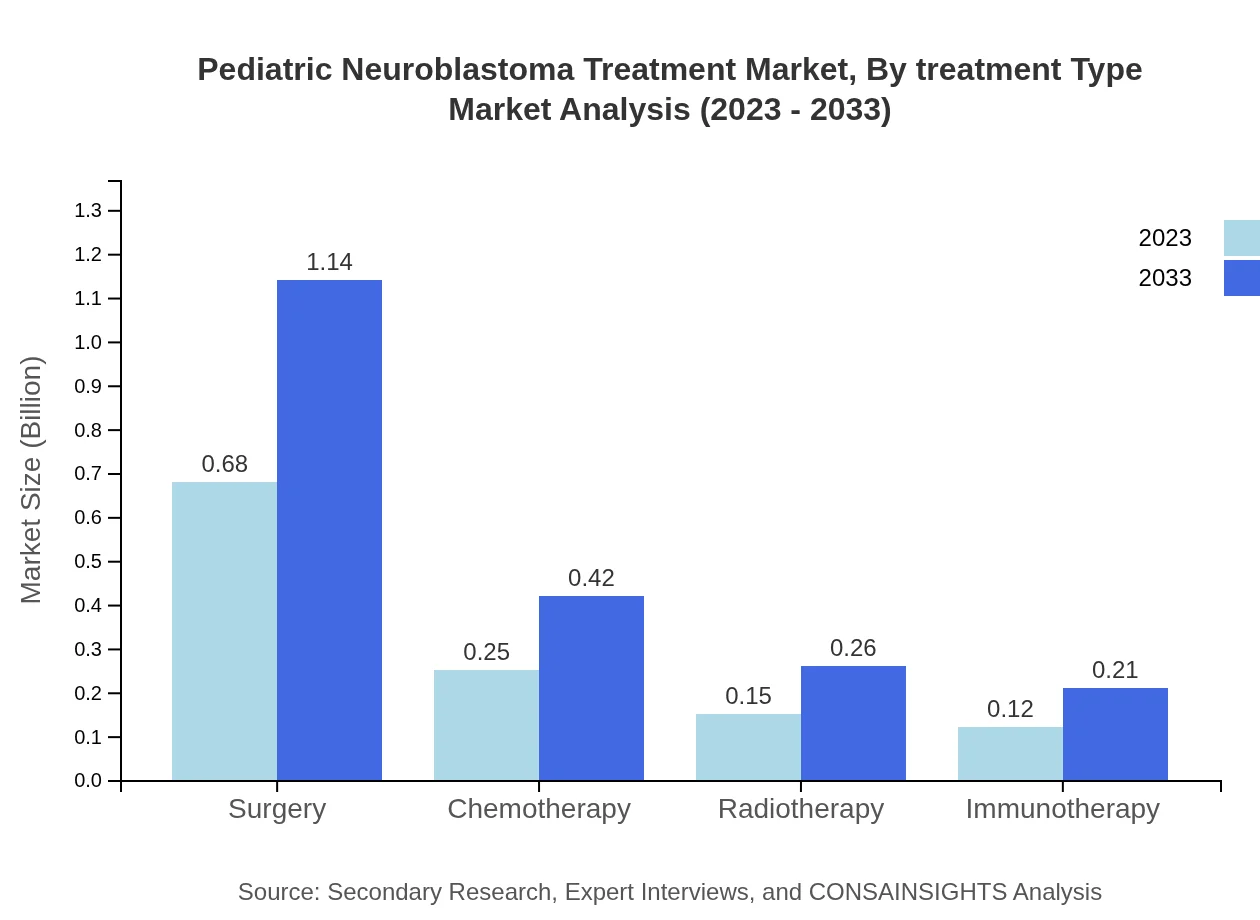
Pediatric Neuroblastoma Treatment Market Analysis By Treatment Type
In terms of treatment types, cytotoxic drugs are predominant in the market, accounting for 65.52% of the share in 2023, projected to remain stable until 2033, rising concurrently in market size from 0.79 billion USD to 1.33 billion USD. Targeted therapy contributes significantly with 20.55% share, showing growth from 0.25 billion USD to 0.42 billion USD. Supportive care comprises 13.93% of the market size, with figures raising from 0.17 to 0.28 billion USD over the same period.
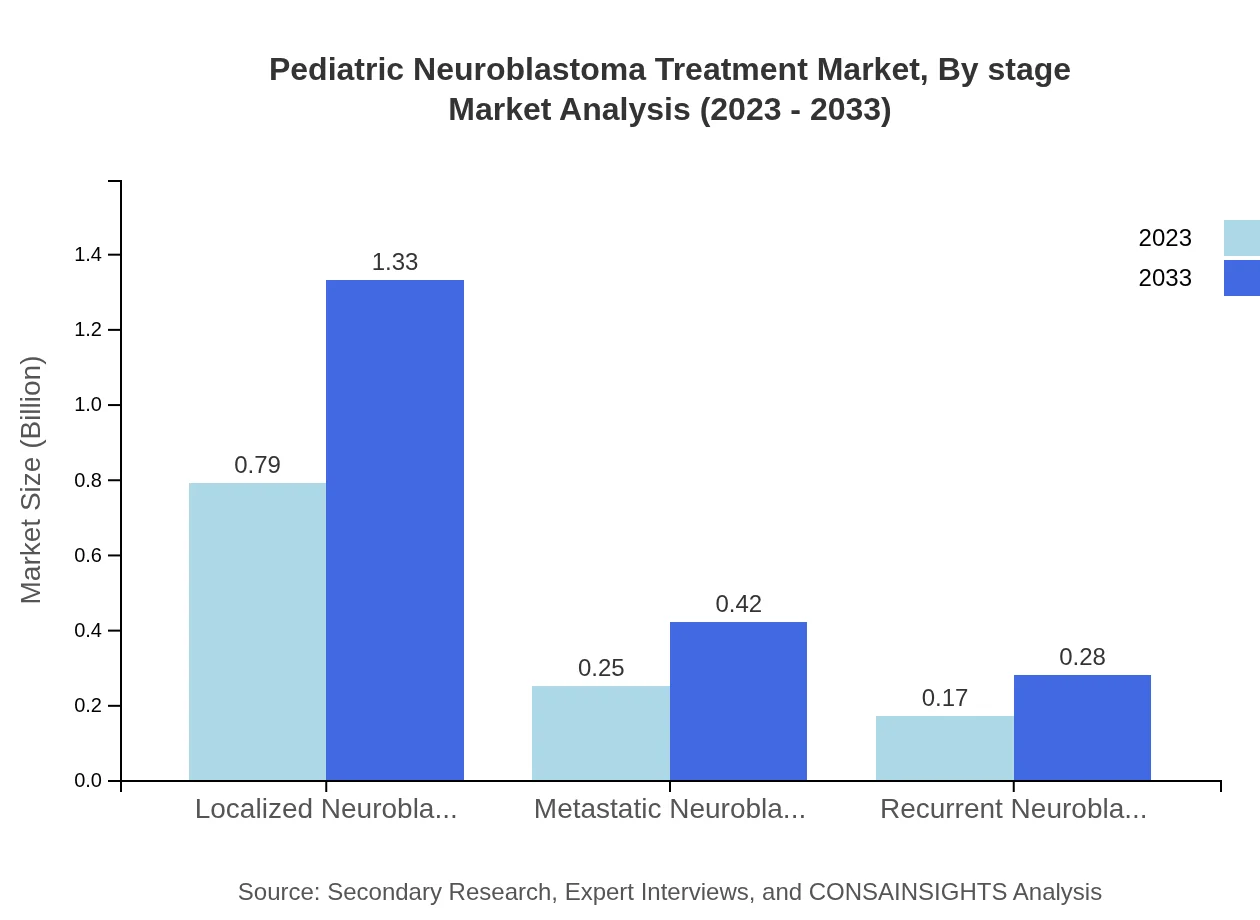
Pediatric Neuroblastoma Treatment Market Analysis By Stage
The market analysis by disease stage highlights that localized neuroblastoma dominates the segment, comprising 65.52% of the market share in 2023 and projected to expand to 1.33 billion USD by 2033. Metastatic neuroblastoma holds 20.55% market share, increasing from 0.25 billion to 0.42 billion USD, while recurrent neuroblastoma contributes 13.93% with growth from 0.17 billion to 0.28 billion USD by 2033.
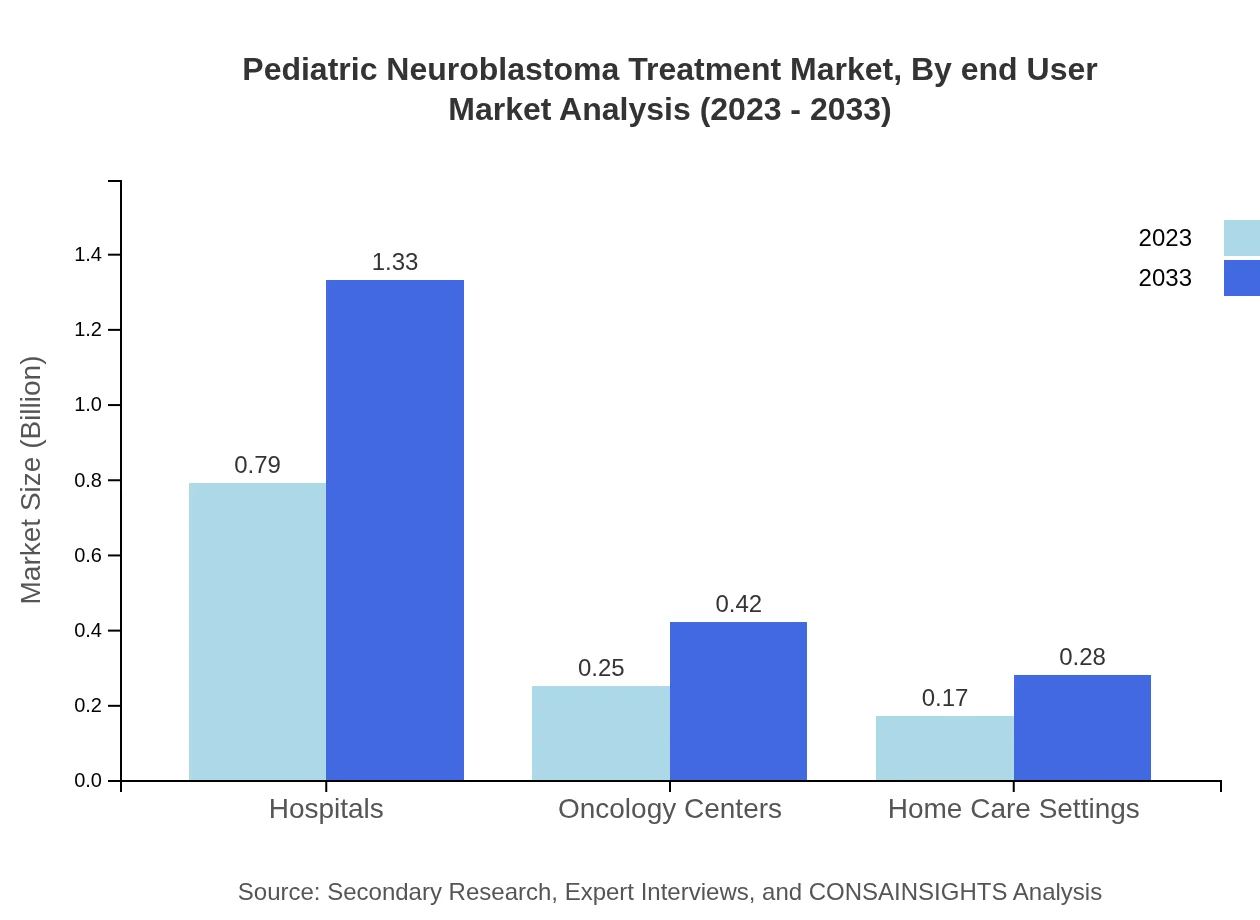
Pediatric Neuroblastoma Treatment Market Analysis By End User
The end-user segmentation reveals that hospitals lead with a market share of 65.52% in 2023, projected to ascend from 0.79 billion to 1.33 billion USD by 2033. Oncology centers represent 20.55% share, with growth from 0.25 billion to 0.42 billion USD, and home care settings account for 13.93%, increasing from 0.17 billion to 0.28 billion USD over the forecast period.
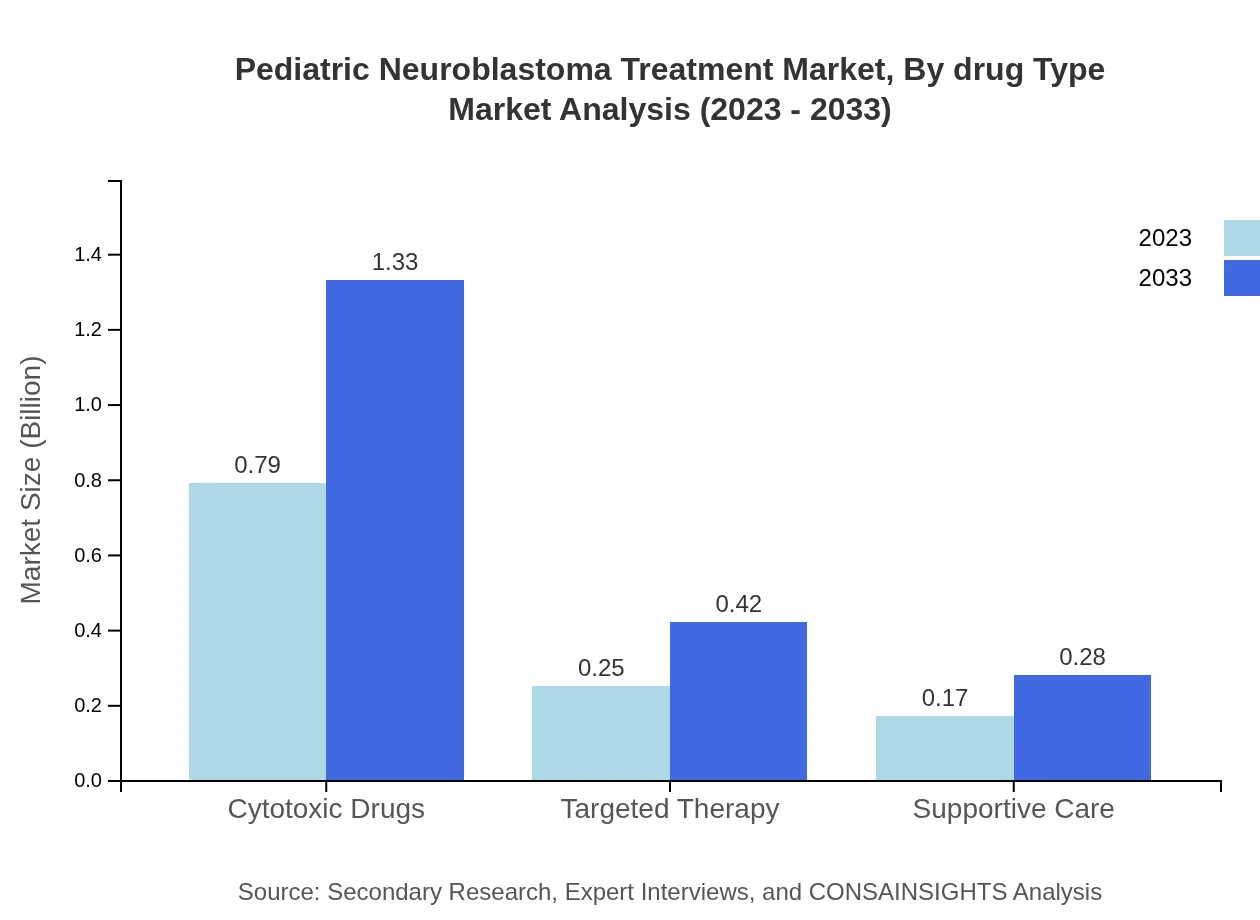
Pediatric Neuroblastoma Treatment Market Analysis By Drug Type
The drug type segmentation outlines cytotoxic drugs as the leading category, comprising 65.52% share in 2023, with a growth trajectory projected from 0.79 billion to 1.33 billion USD by 2033. Targeted therapy follows at 20.55%, increasing from 0.25 billion to 0.42 billion USD, while supportive care drugs hold a 13.93% share, expected to rise from 0.17 billion to 0.28 billion USD.
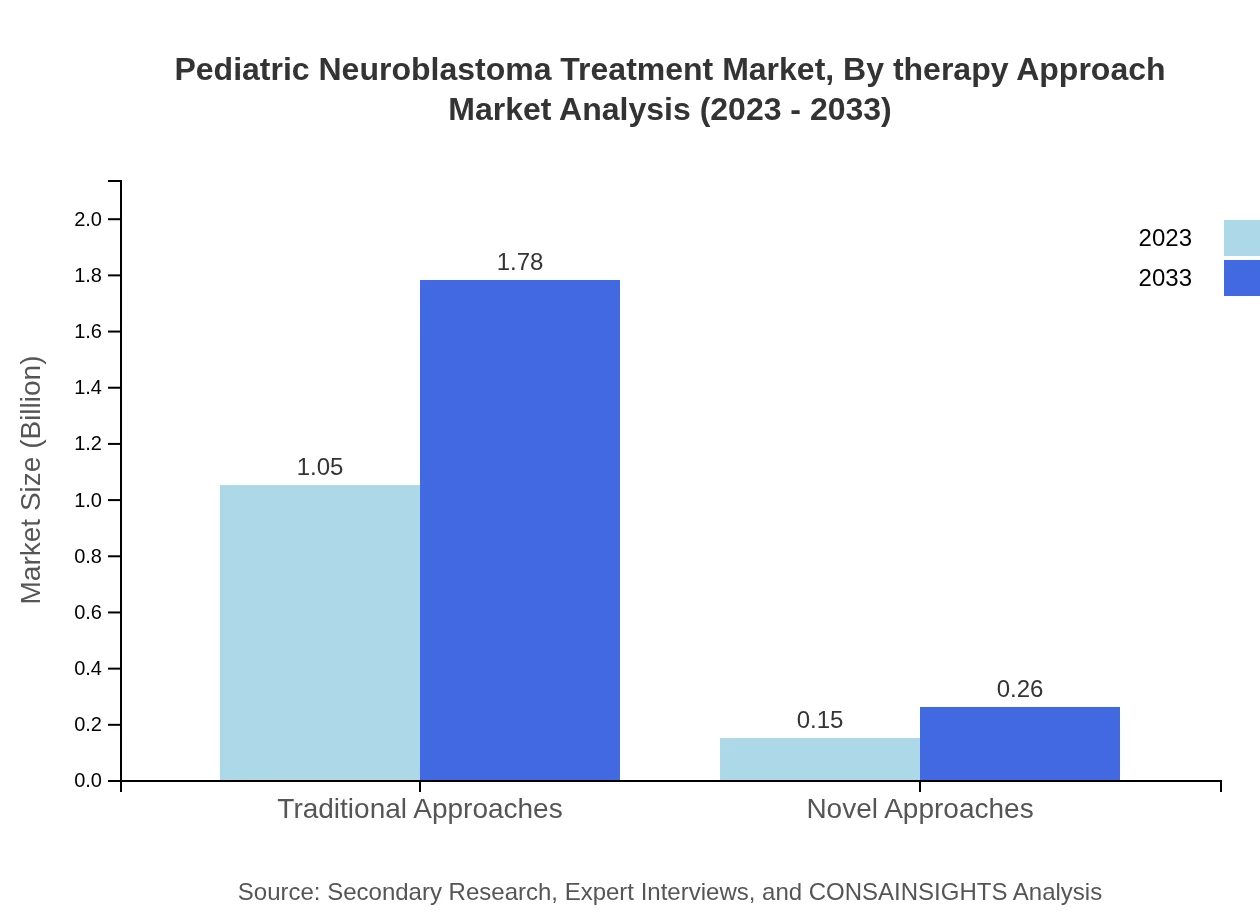
Pediatric Neuroblastoma Treatment Market Analysis By Therapy Approach
A breakdown by therapy approach indicates traditional therapies dominate with an 87.36% market share, growing from 1.05 billion USD to 1.78 billion USD. Novel approaches are gaining traction, representing 12.64% of the market, moving from 0.15 billion to 0.26 billion USD throughout the forecast period.
Pediatric Neuroblastoma Treatment Market Trends and Future Forecast
Tell us your focus area and get a customized research report.
Global Market Leaders and Top Companies in Pediatric Neuroblastoma Treatment Industry
Novartis AG:
A leading global healthcare company, Novartis specializes in innovative medicines, including oncology therapy solutions for pediatric neuroblastoma. Their commitment to research has positioned them as a key player in the field.Roche Holding AG:
Roche focuses on personalized healthcare and provides a range of treatments for neuroblastoma, leveraging advanced research methodologies. Their initiatives in immunotherapy showcase their dedication to improving survival rates in pediatric patients.Bristol Myers Squibb Co.:
Bristol Myers Squibb develops and delivers innovative medicines for various diseases, including neuroblastoma, focusing heavily on combination therapies and novel drug development.Eli Lilly and Company:
Eli Lilly’s efforts in pediatric oncology include new drug development for neuroblastoma treatments, merging both traditional and novel approaches to enhance patient outcomes.Sanofi:
Sanofi is committed to addressing unmet medical needs in oncology, including pediatric neuroblastoma, by investing in R&D and improving access to advanced treatment options.We're grateful to work with incredible clients.









FAQs
What is the market size of pediatric Neuroblastoma Treatment?
The pediatric neuroblastoma treatment market is projected to reach approximately $1.2 billion by 2033, growing at a CAGR of 5.3%. The increasing prevalence of neuroblastoma among children is significantly contributing to market growth.
What are the key market players or companies in this pediatric Neuroblastoma Treatment industry?
Key players in the pediatric neuroblastoma treatment industry include large pharmaceutical companies, biotechnology firms, and specialized healthcare providers. Notable companies may include those focusing on innovative treatments and clinical trials specific to neuroblastoma.
What are the primary factors driving the growth in the pediatric Neuroblastoma Treatment industry?
The growth of the pediatric neuroblastoma treatment industry is driven by factors such as advancements in treatment options, increased awareness of pediatric cancers, ongoing research, and the development of targeted therapies that improve patient outcomes.
Which region is the fastest Growing in the pediatric Neuroblastoma Treatment?
The Asia-Pacific region is emerging as the fastest-growing market for pediatric neuroblastoma treatment. The market is expected to grow from $0.23 billion in 2023 to $0.39 billion by 2033, fueled by rising healthcare investments and improved access to treatment.
Does ConsaInsights provide customized market report data for the pediatric Neuroblastoma Treatment industry?
Yes, ConsaInsights provides customized market report data tailored to specific needs within the pediatric neuroblastoma treatment industry, ensuring clients receive the most relevant insights and analysis for their objectives.
What deliverables can I expect from this pediatric Neuroblastoma Treatment market research project?
Clients can expect comprehensive deliverables that include market size analysis, growth forecasts, competitive landscape assessments, regional insights, segment data, and strategic recommendations tailored for the pediatric neuroblastoma treatment market.
What are the market trends of pediatric Neuroblastoma Treatment?
Current market trends in pediatric neuroblastoma treatment include a shift towards personalized medicine, increased utilization of immunotherapies, a focus on novel drug development, and an emphasis on early detection and intervention strategies.