Pediatric Respiratory Disease Therapeutics Market Report
Published Date: 31 January 2026 | Report Code: pediatric-respiratory-disease-therapeutics
Pediatric Respiratory Disease Therapeutics Market Size, Share, Industry Trends and Forecast to 2033
This report provides a comprehensive analysis of the Pediatric Respiratory Disease Therapeutics market, covering key market trends, growth forecasts, and regional insights for the period 2023 to 2033.
| Metric | Value |
|---|---|
| Study Period | 2023 - 2033 |
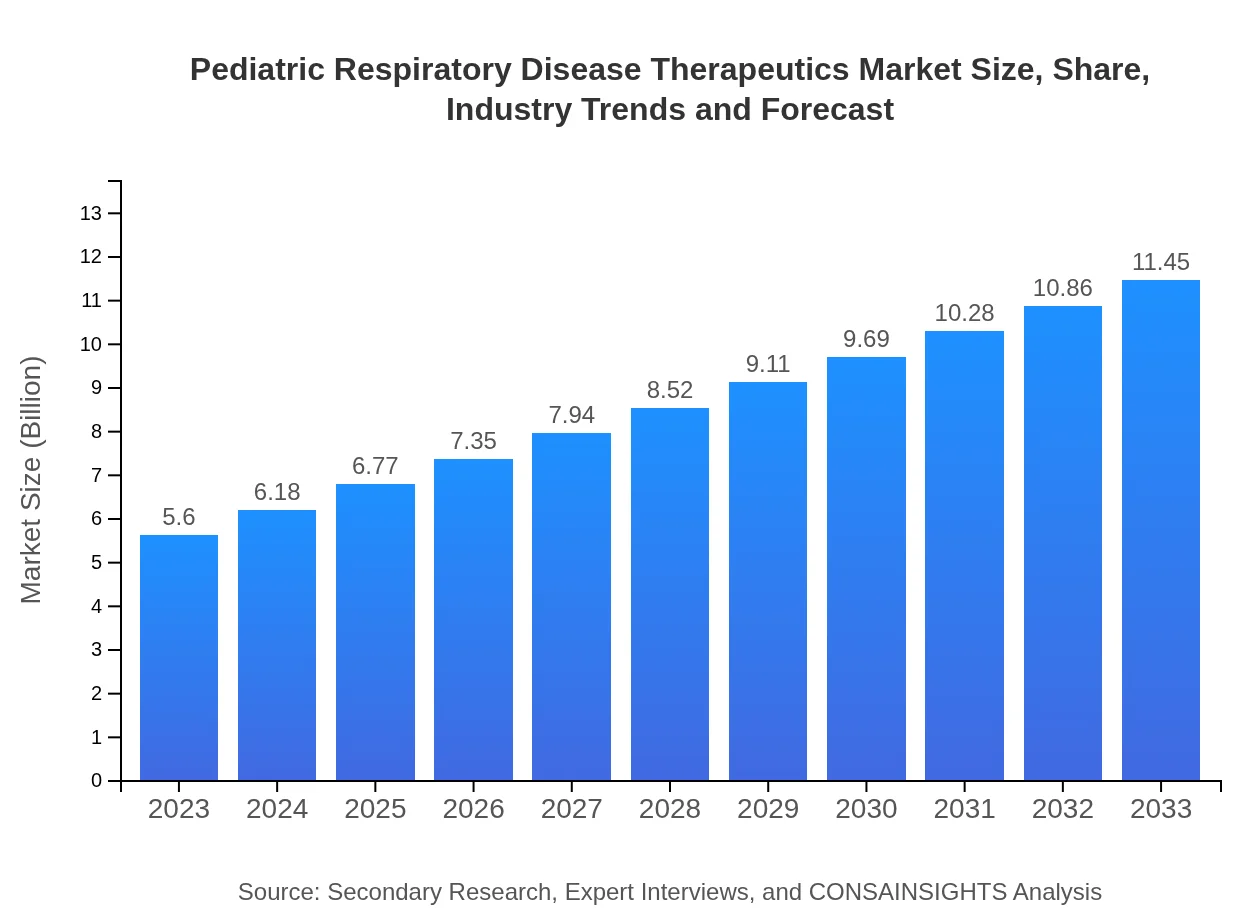
| 2023 Market Size | $5.60 Billion |
| CAGR (2023-2033) | 7.2% |
| 2033 Market Size | $11.45 Billion |
| Top Companies | Pfizer , GlaxoSmithKline, Roche, Novartis, AstraZeneca |
| Last Modified Date | 31 January 2026 |
Pediatric Respiratory Disease Therapeutics Market Overview
Customize Pediatric Respiratory Disease Therapeutics Market Report market research report
- ✔ Get in-depth analysis of Pediatric Respiratory Disease Therapeutics market size, growth, and forecasts.
- ✔ Understand Pediatric Respiratory Disease Therapeutics's regional dynamics and industry-specific trends.
- ✔ Identify potential applications, end-user demand, and growth segments in Pediatric Respiratory Disease Therapeutics
What is the Market Size & CAGR of Pediatric Respiratory Disease Therapeutics market in 2023?
Pediatric Respiratory Disease Therapeutics Industry Analysis
Pediatric Respiratory Disease Therapeutics Market Segmentation and Scope
Tell us your focus area and get a customized research report.
Pediatric Respiratory Disease Therapeutics Market Analysis Report by Region
Europe Pediatric Respiratory Disease Therapeutics Market Report:
The European market is projected to grow from $1.89 billion in 2023 to $3.87 billion by 2033, driven by increasing awareness about respiratory diseases and supportive healthcare policies aimed at improving children’s health outcomes.Asia Pacific Pediatric Respiratory Disease Therapeutics Market Report:
In the Asia Pacific region, the Pediatric Respiratory Disease Therapeutics market was valued at approximately $0.95 billion in 2023 and is expected to reach $1.93 billion by 2033. Growing pollution levels, urbanization, and a rise in allergic conditions are driving the demand for respiratory therapies.North America Pediatric Respiratory Disease Therapeutics Market Report:
North America leads the Pediatric Respiratory Disease Therapeutics market with a valuation of $1.96 billion in 2023, expected to grow to $4.01 billion by 2033. This region is home to advanced healthcare infrastructure and significant investments in R&D, enhancing therapeutic offerings for pediatric patients.South America Pediatric Respiratory Disease Therapeutics Market Report:
The South American Pediatric Respiratory Disease Therapeutics market was valued at $0.07 billion in 2023, with projections indicating a growth to $0.15 billion by 2033. Increasing healthcare access and improvements in disease management are key factors driving growth in this region.Middle East & Africa Pediatric Respiratory Disease Therapeutics Market Report:
The Middle East and Africa Pediatric Respiratory Disease Therapeutics market is expected to increase from $0.73 billion in 2023 to $1.49 billion by 2033, with increasing investments in healthcare services and rising awareness about pediatric health initiatives.Tell us your focus area and get a customized research report.
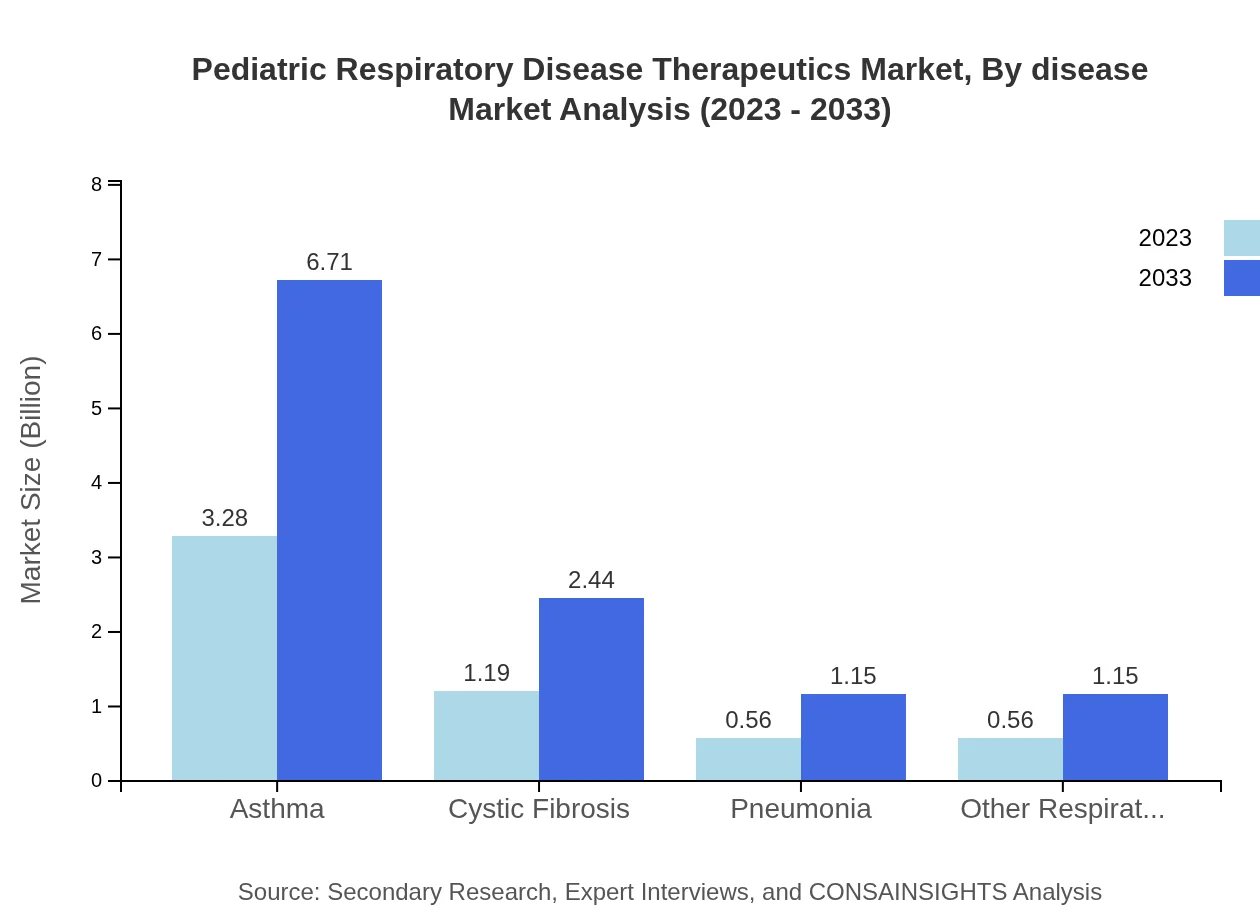
Pediatric Respiratory Disease Therapeutics Market Analysis By Disease
Asthma remains the dominant segment of the Pediatric Respiratory Disease Therapeutics market, valued at $3.28 billion in 2023 and projected to grow to $6.71 billion by 2033, capturing a market share of 58.63% throughout the forecast period. Cystic Fibrosis and Pneumonia are also significant, with market sizes rising from $0.56 billion and $1.19 billion respectively in 2023 to $1.15 billion and $2.44 billion by 2033.
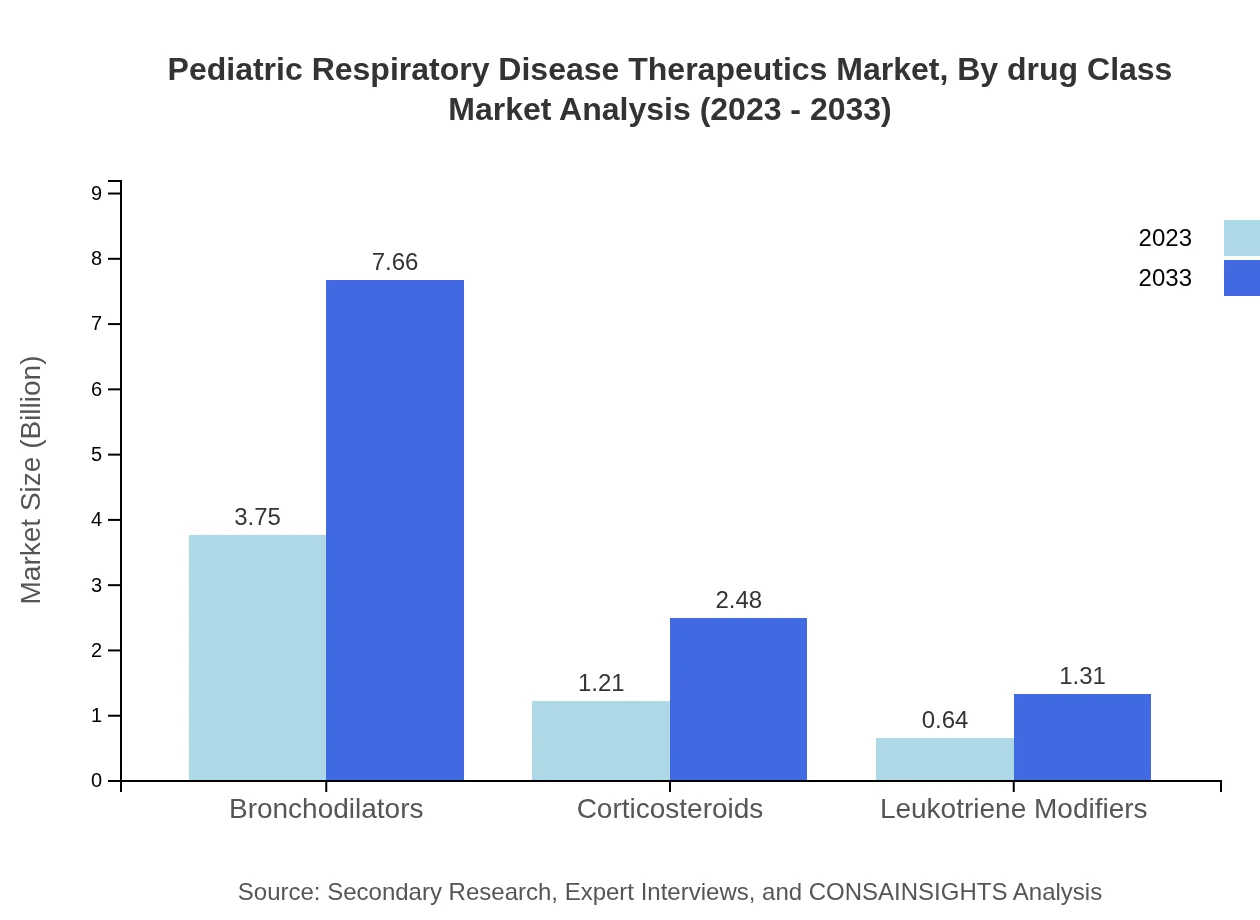
Pediatric Respiratory Disease Therapeutics Market Analysis By Drug Class
Bronchodilators lead the therapeutic options, valued at $3.75 billion in 2023 and expected to grow to $7.66 billion by 2033. Corticosteroids and Leukotriene Modifiers represent notable shares as well, growing steadily in alignment with increasing asthma and allergic disease diagnoses.
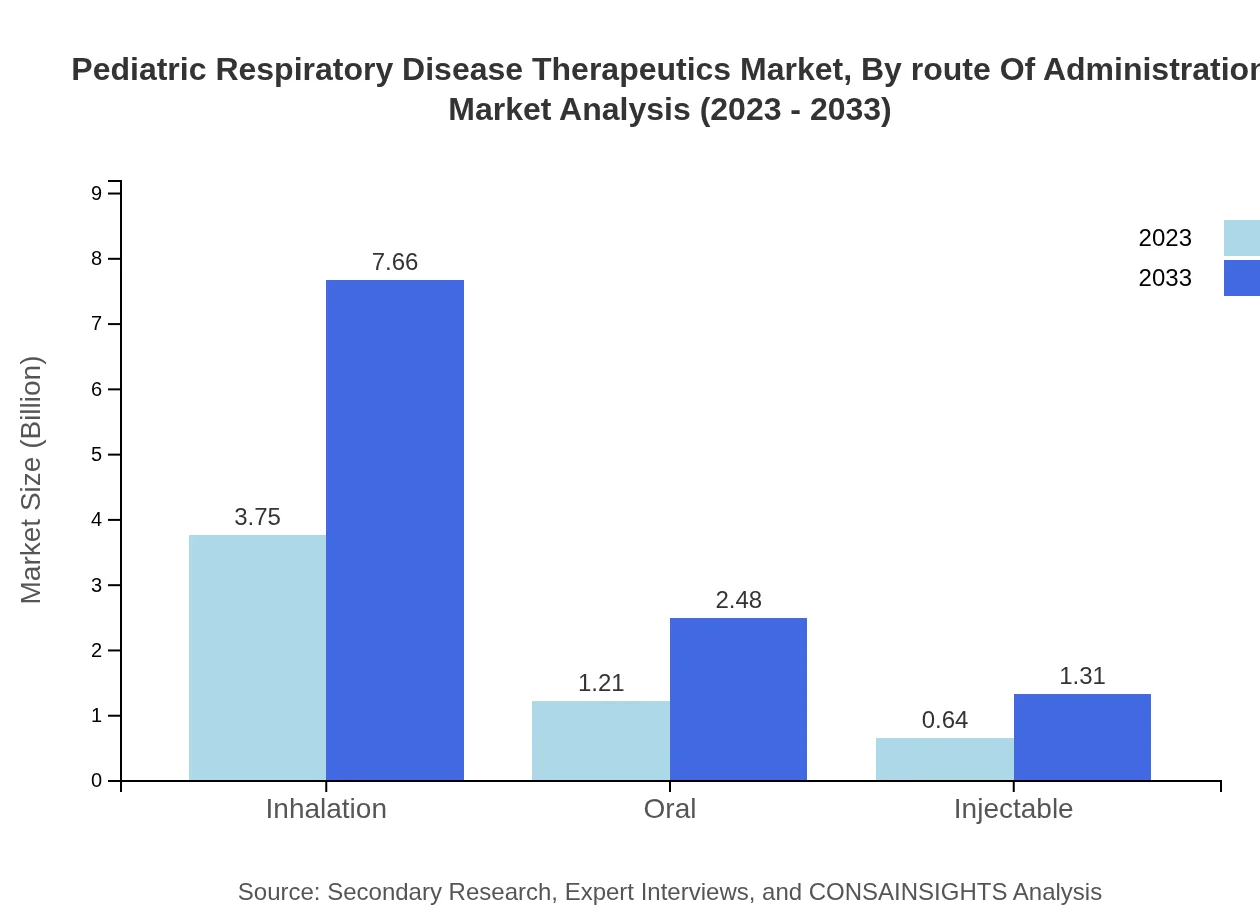
Pediatric Respiratory Disease Therapeutics Market Analysis By Route Of Administration
Inhalation methods dominate the market, accounting for $3.75 billion in 2023. Oral administration follows, valued at $1.21 billion. Innovative drug delivery systems are being explored to enhance patient compliance and drug absorption, particularly among pediatric populations.
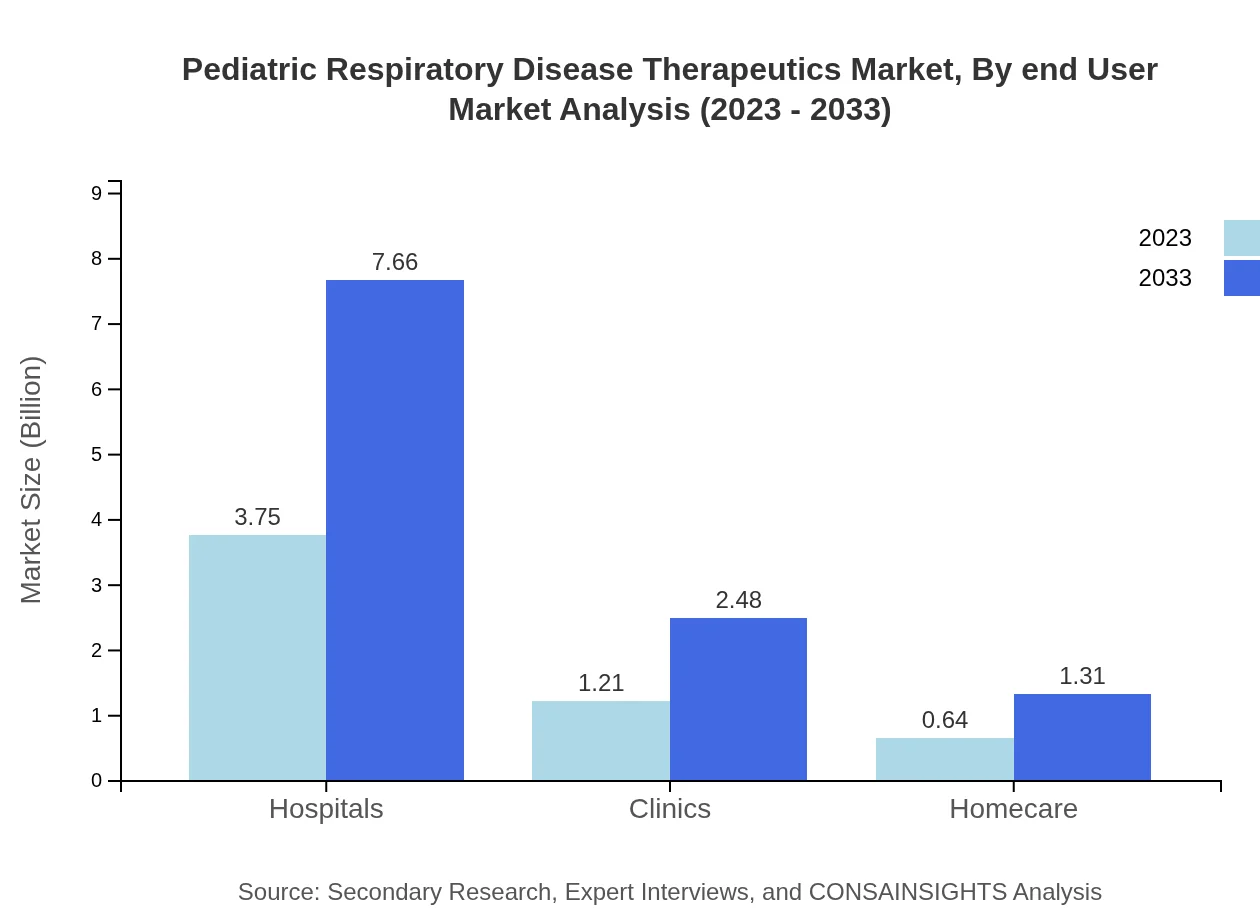
Pediatric Respiratory Disease Therapeutics Market Analysis By End User
Hospitals remain the key end-user segment, generating $3.75 billion in 2023, with clinics and homecare settings also growing, thereby allowing for more comprehensive management of respiratory diseases in children.
Pediatric Respiratory Disease Therapeutics Market Analysis By Competitive Landscape
The competitive landscape is characterized by the presence of key players focusing on R&D and strategic partnerships, with a focus on developing innovative therapies and addressing unmet medical needs in the pediatric population.
Pediatric Respiratory Disease Therapeutics Market Trends and Future Forecast
Tell us your focus area and get a customized research report.
Global Market Leaders and Top Companies in Pediatric Respiratory Disease Therapeutics Industry
Pfizer :
Pfizer is a leading global pharmaceutical company known for its wide range of medications, including therapies for pediatric respiratory diseases.GlaxoSmithKline:
GlaxoSmithKline specializes in developing innovative therapeutic solutions for respiratory health, emphasizing pediatric applications.Roche:
Roche is recognized for its advanced biopharmaceuticals, particularly in the treatment of cystic fibrosis and related conditions.Novartis:
Novartis has a significant portfolio in respiratory therapeutics, focusing on evidence-based treatments for pediatric patients.AstraZeneca:
AstraZeneca is a major player in the development of inhaled therapies and is committed to advancing care for children with respiratory diseases.We're grateful to work with incredible clients.









FAQs
What is the market size of pediatric Respiratory Disease Therapeutics?
The global pediatric respiratory disease therapeutics market is projected to reach approximately $5.6 billion by 2033, with a compound annual growth rate (CAGR) of 7.2%. This growth indicates a strong demand for effective treatments in this segment.
What are the key market players or companies in the pediatric Respiratory Disease Therapeutics industry?
Key players in the pediatric respiratory disease therapeutics market include major pharmaceutical and biotechnology firms that specialize in developing treatments such as bronchodilators and corticosteroids, which cater specifically to children’s respiratory conditions.
What are the primary factors driving the growth in the pediatric respiratory disease therapeutics industry?
The growth of the pediatric respiratory disease therapeutics market is primarily driven by rising incidences of respiratory diseases in children, advancements in treatment options, increased healthcare spending, and a focus on research and development in pediatric medicine.
Which region is the fastest Growing in the pediatric Respiratory Disease Therapeutics?
North America is the fastest-growing region in the pediatric respiratory disease therapeutics market, with market projections increasing from $1.96 billion in 2023 to $4.01 billion by 2033, driven by enhanced healthcare infrastructure and awareness.
Does ConsaInsights provide customized market report data for the pediatric respiratory disease Therapeutics industry?
Yes, ConsaInsights offers customized market report data tailored to specific needs within the pediatric respiratory disease therapeutics industry, allowing stakeholders to access relevant insights based on unique market parameters.
What deliverables can I expect from this pediatric Respiratory Disease Therapeutics market research project?
Deliverables from this market research project include comprehensive market analyses, region-specific forecasts, segment data, competitive landscape insights, and tailored recommendations to support strategic decision-making.
What are the market trends of pediatric Respiratory Disease Therapeutics?
Current market trends in pediatric respiratory disease therapeutics include a shift towards personalized medicine, greater availability of advanced therapies, increased focus on preventive care, and the development of innovative drug delivery methods.