Pediatric Vaccines Market Report
Published Date: 31 January 2026 | Report Code: pediatric-vaccines
Pediatric Vaccines Market Size, Share, Industry Trends and Forecast to 2033
This report provides a comprehensive analysis of the Pediatric Vaccines market from 2023 to 2033. It covers market dynamics, trends, segmentation, regional insights, and future forecasts, delivering key insights for stakeholders and decision-makers in the healthcare industry.
| Metric | Value |
|---|---|
| Study Period | 2023 - 2033 |
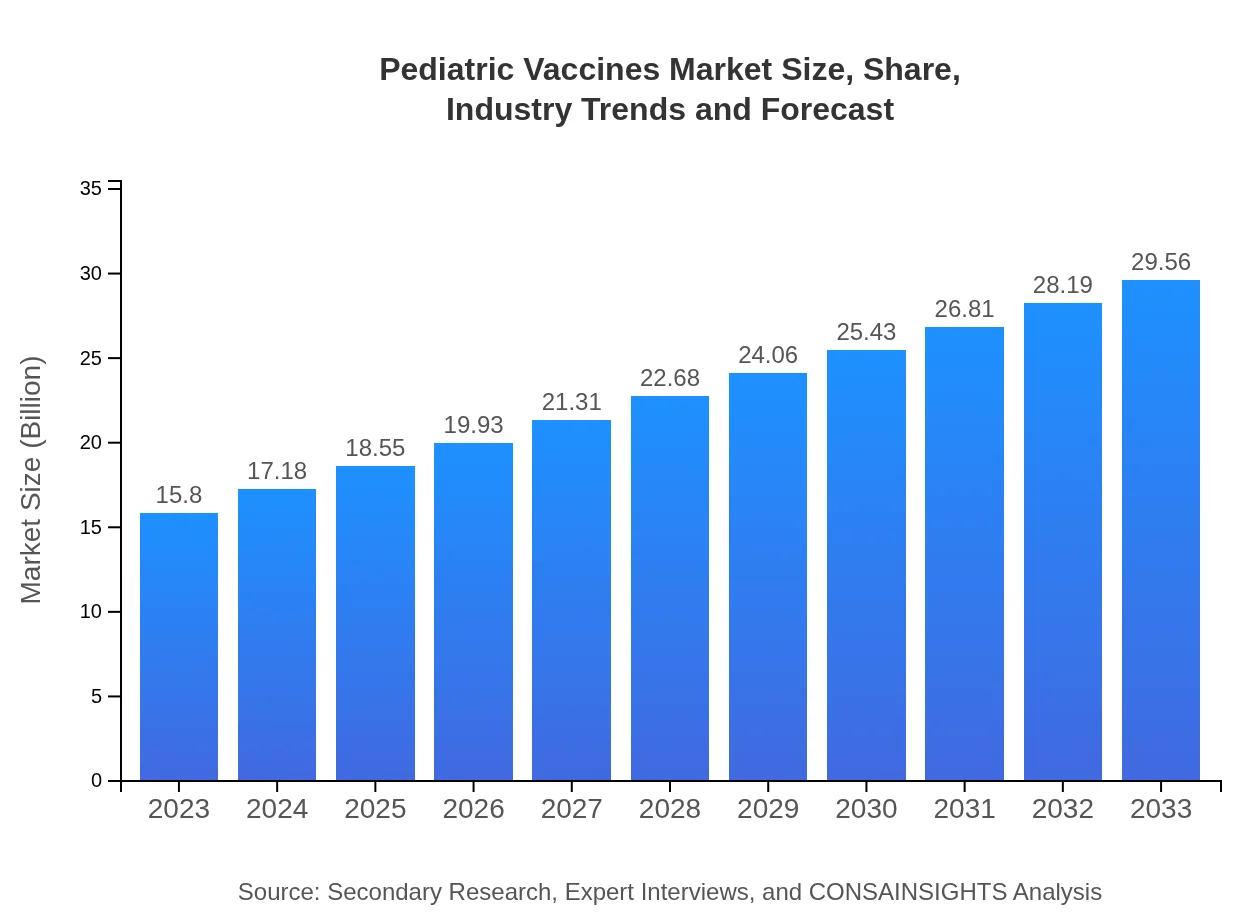
| 2023 Market Size | $15.80 Billion |
| CAGR (2023-2033) | 6.3% |
| 2033 Market Size | $29.56 Billion |
| Top Companies | Pfizer , Merck & Co., GlaxoSmithKline, Sanofi Pasteur, Bharat Biotech |
| Last Modified Date | 31 January 2026 |
Pediatric Vaccines Market Overview
Customize Pediatric Vaccines Market Report market research report
- ✔ Get in-depth analysis of Pediatric Vaccines market size, growth, and forecasts.
- ✔ Understand Pediatric Vaccines's regional dynamics and industry-specific trends.
- ✔ Identify potential applications, end-user demand, and growth segments in Pediatric Vaccines
What is the Market Size & CAGR of Pediatric Vaccines Market in 2033?
Pediatric Vaccines Industry Analysis
Pediatric Vaccines Market Segmentation and Scope
Tell us your focus area and get a customized research report.
Pediatric Vaccines Market Analysis Report by Region
Europe Pediatric Vaccines Market Report:
The European market will grow from $4.45 billion in 2023 to $8.32 billion by 2033. Increased demand for preventive healthcare and stringent regulatory frameworks facilitate trust and uptake of vaccinations. Healthcare reforms are also promoting accessibility across member states.Asia Pacific Pediatric Vaccines Market Report:
The Asia Pacific region's Pediatric Vaccines market is projected to grow from $3.36 billion in 2023 to $6.29 billion by 2033, driven by increasing public health initiatives and rising immunization awareness. The region is witnessing substantial investment in vaccine infrastructure, contributing to enhanced distribution capabilities and accessibility.North America Pediatric Vaccines Market Report:
North America's Pediatric Vaccines market is projected to rise from $5.33 billion in 2023 to $9.98 billion by 2033, reflecting strong healthcare systems, high vaccination rates, and innovative research initiatives. The ongoing development of new vaccines is also anticipated to drive market advancements.South America Pediatric Vaccines Market Report:
In South America, the market is expected to expand from $0.75 billion in 2023 to $1.41 billion by 2033. Governments are actively promoting vaccination programs to combat childhood diseases, and the region benefits from international cooperation and funding aimed at improving healthcare services.Middle East & Africa Pediatric Vaccines Market Report:
The Middle East and Africa region's market is expected to increase from $1.91 billion in 2023 to $3.57 billion by 2033, propelled by investments in healthcare infrastructure and collaborative efforts to enhance healthcare delivery systems, particularly in remote areas.Tell us your focus area and get a customized research report.
Pediatric Vaccines Market Analysis By Product
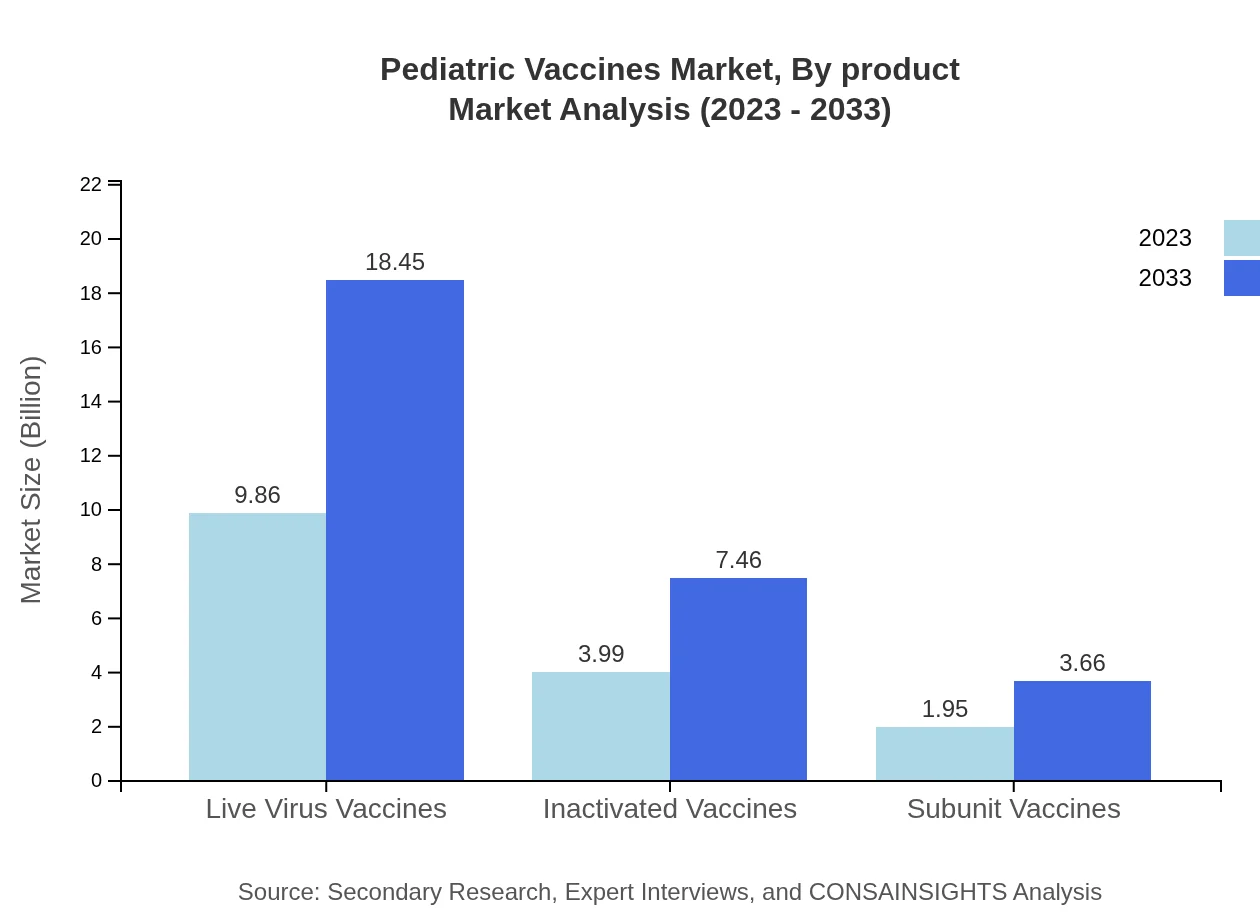
The Pediatric Vaccines market segments by product type include live virus vaccines, inactivated vaccines, and subunit vaccines. Live virus vaccines dominate with a market size projected to grow from $9.86 billion in 2023 to $18.45 billion in 2033, representing a 62.4% share throughout the forecast period. Inactivated vaccines will increase from $3.99 billion to $7.46 billion, holding a significant market share of 25.23%, while subunit vaccines will grow from $1.95 billion to $3.66 billion, contributing a 12.37% share.
Pediatric Vaccines Market Analysis By Age Group
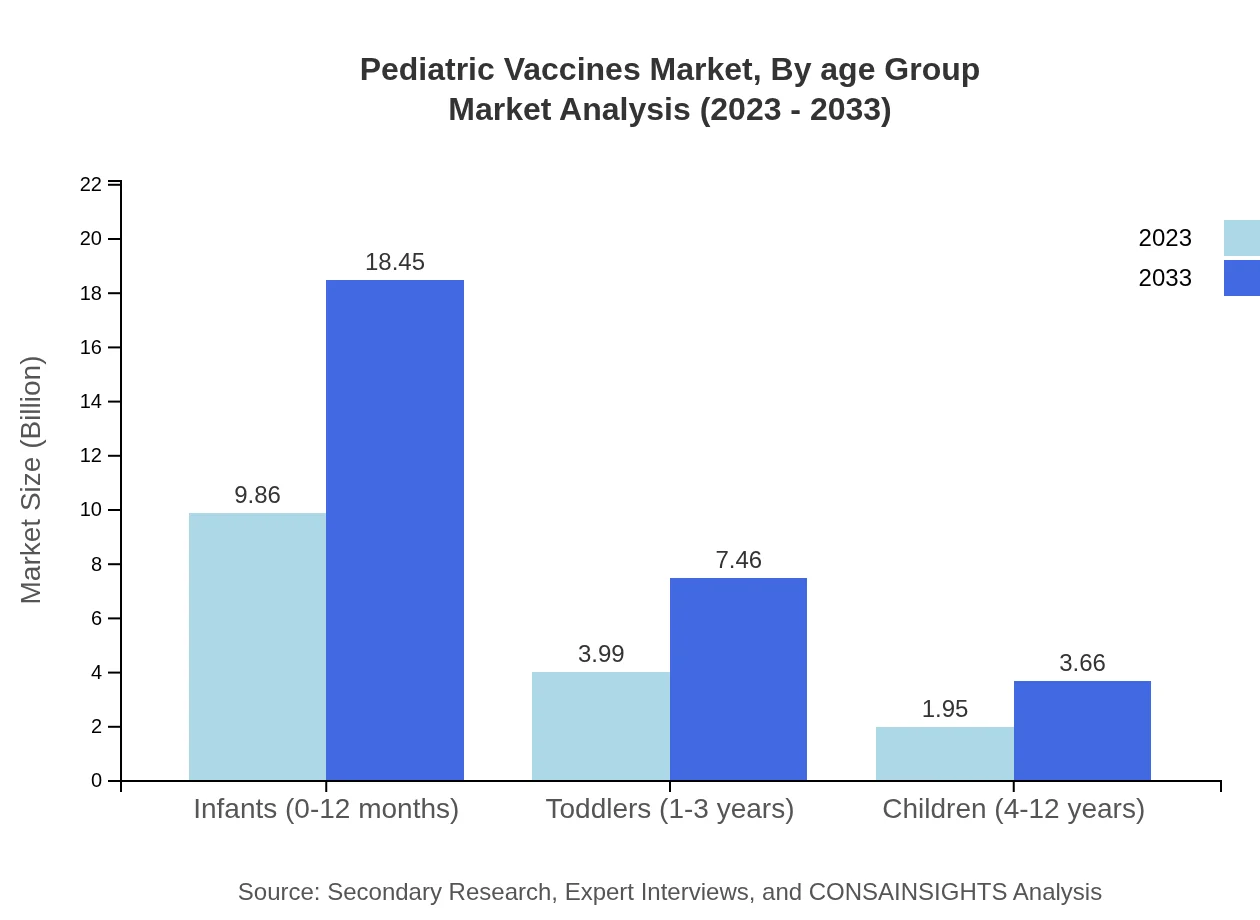
The market by age group segments into infants (0-12 months), toddlers (1-3 years), and children (4-12 years). Infants represent the largest group, with a market size projected to reach $9.86 billion by 2033, maintaining a 62.4% market share. Toddlers are expected to grow from $3.99 billion to $7.46 billion, capturing 25.23% of the market. Children aged 4-12 years will increase from $1.95 billion to $3.66 billion, representing a 12.37% share.
Pediatric Vaccines Market Analysis By Administration Route
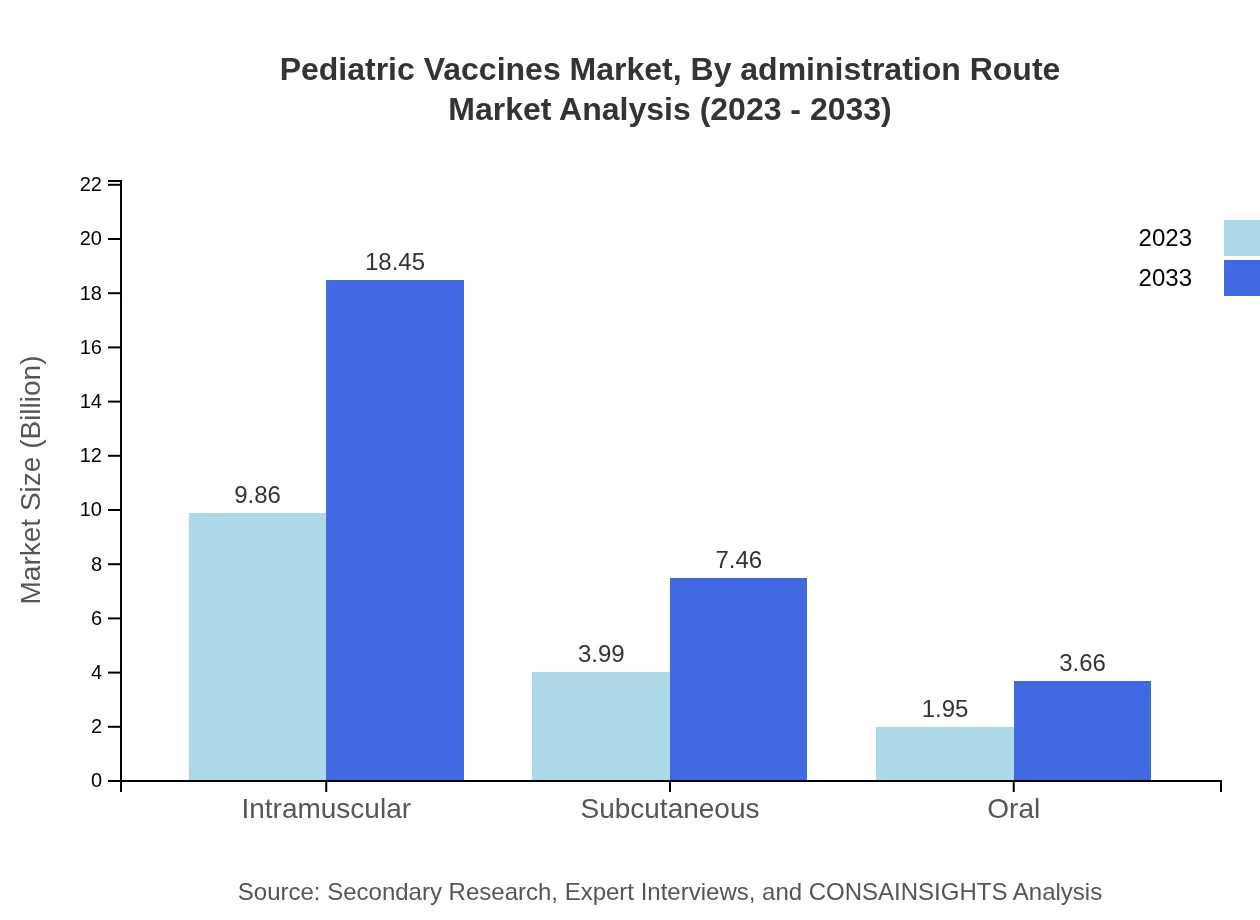
Pediatric Vaccines are administered primarily via intramuscular, subcutaneous, and oral routes. Intramuscular administration leads the route segment, with growth from $9.86 billion to $18.45 billion, maintaining a 62.4% market share. Subcutaneous injections will increase from $3.99 billion to $7.46 billion, holding a share of 25.23%, while oral administration will rise from $1.95 billion to $3.66 billion, securing a 12.37% share.
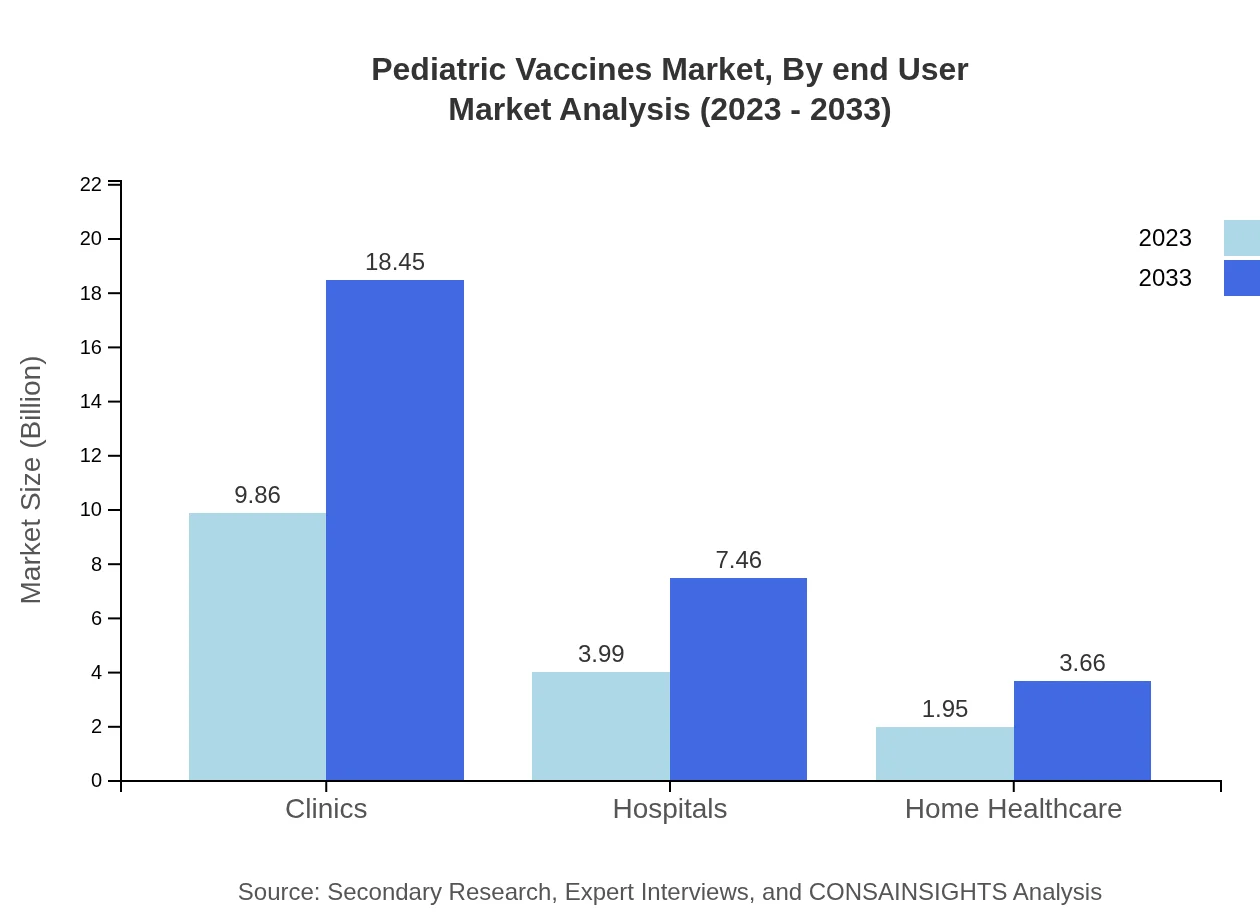
Pediatric Vaccines Market Analysis By End User
By end-user, the Pediatric Vaccines market includes hospitals, clinics, and home healthcare. Clinics dominate the sector with sizable growth from $9.86 billion to $18.45 billion, holding a 62.4% share. Hospitals will expand from $3.99 billion to $7.46 billion, representing 25.23% share, while home healthcare services grow from $1.95 billion to $3.66 billion, retaining a 12.37% share.
Pediatric Vaccines Market Trends and Future Forecast
Tell us your focus area and get a customized research report.
Global Market Leaders and Top Companies in Pediatric Vaccines Industry
Pfizer :
Pfizer leads the Pediatric Vaccines market with a diverse portfolio, including vaccines for pneumococcal disease and meningococcal infections, contributing significantly to public health through innovative vaccine solutions.Merck & Co.:
Merck & Co. is renowned for its comprehensive vaccine lineup, including the MMR vaccine and various HPV vaccines, establishing a strong presence in the pediatric vaccination landscape.GlaxoSmithKline:
GlaxoSmithKline is recognized for its commitment to infectious disease prevention through vaccines, focusing on innovative solutions to enhance immunization rates among children.Sanofi Pasteur:
Sanofi Pasteur is a global leader in vaccine production, particularly in pediatric vaccines, ensuring wide accessibility to essential immunization programs worldwide.Bharat Biotech:
Bharat Biotech, a significant player in the emerging markets, contributes to pediatric vaccine delivery in India and other countries, particularly focusing on affordable vaccine solutions.We're grateful to work with incredible clients.









FAQs
What is the market size of pediatric vaccines?
The pediatric vaccines market is valued at approximately $15.8 billion in 2023, with a projected CAGR of 6.3% through 2033. This growth indicates a rising demand for immunization services globally.
What are the key market players or companies in the pediatric vaccines industry?
Key players in the pediatric vaccines market include major pharmaceutical companies such as GlaxoSmithKline, Merck & Co., Pfizer, Sanofi, and Johnson & Johnson, which are pivotal in vaccine development and distribution.
What are the primary factors driving the growth in the pediatric vaccines industry?
The growth of the pediatric vaccines market is driven by increased awareness of vaccination benefits, government initiatives, rising incidences of infectious diseases, and advancements in vaccine technology.
Which region is the fastest Growing in the pediatric vaccines market?
The Asia Pacific region is anticipated to be the fastest-growing in the pediatric vaccines market, increasing from $3.36 billion in 2023 to $6.29 billion by 2033, reflecting heightened immunization efforts.
Does ConsaInsights provide customized market report data for the pediatric vaccines industry?
Yes, ConsaInsights offers customized market report data tailored to your specific needs in the pediatric vaccines industry, ensuring you receive the most relevant and actionable insights.
What deliverables can I expect from this pediatric vaccines market research project?
From this market research project, you can expect comprehensive reports detailing market size, growth forecasts, segment analysis, competitive landscape, and regional insights specific to the pediatric vaccines market.
What are the market trends of pediatric vaccines?
Current trends in the pediatric vaccines market include increasing focus on combination vaccines, growing adoption of electronic health records for tracking immunizations, and a shift towards developing more effective yet cost-efficient vaccines.