Peptide Therapeutics Market Report
Published Date: 31 January 2026 | Report Code: peptide-therapeutics
Peptide Therapeutics Market Size, Share, Industry Trends and Forecast to 2033
This report delves into the Peptide Therapeutics market, providing comprehensive insights into market size, growth forecasts for 2023-2033, regional analyses, and trends. It outlines key segments and leaders influencing the industry landscape.
| Metric | Value |
|---|---|
| Study Period | 2023 - 2033 |
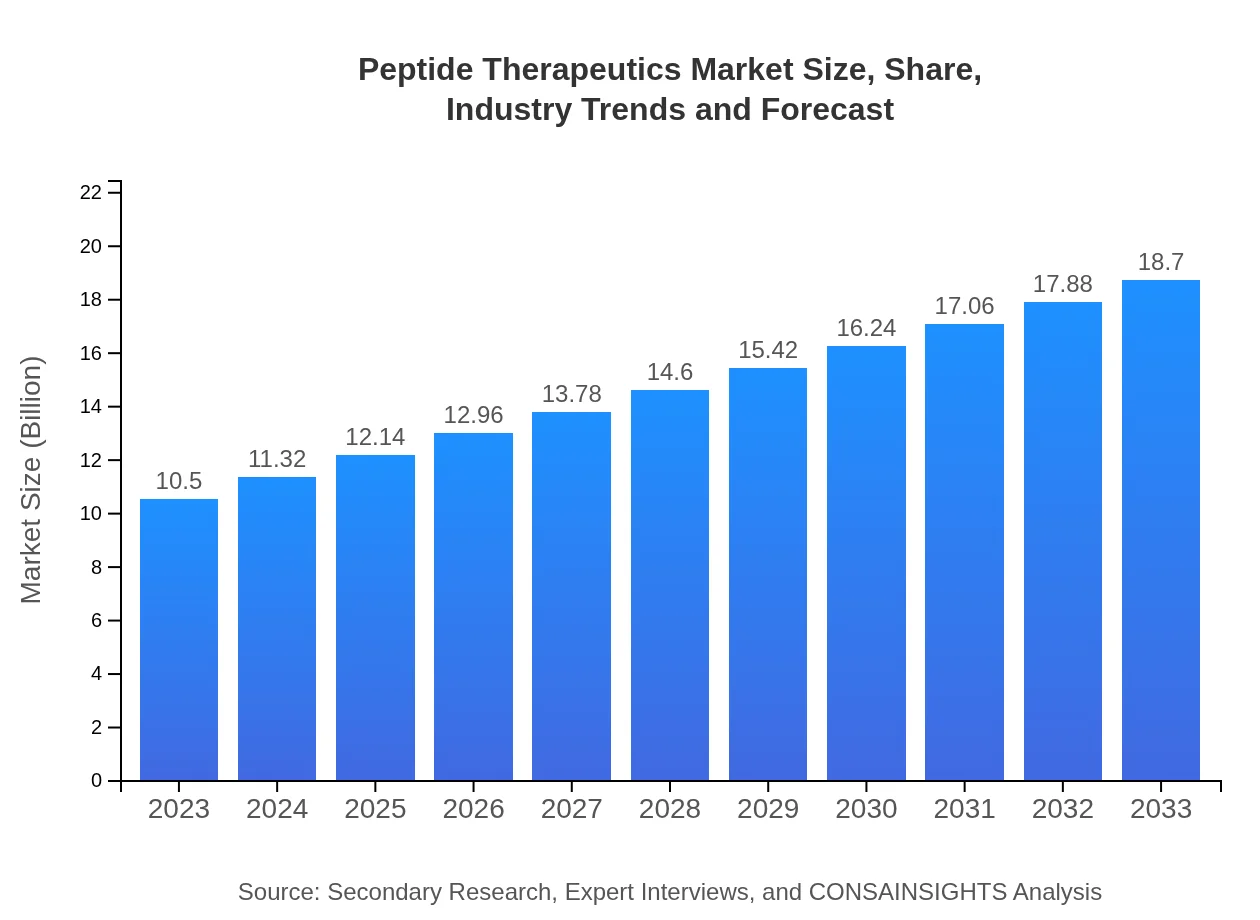
| 2023 Market Size | $10.50 Billion |
| CAGR (2023-2033) | 5.8% |
| 2033 Market Size | $18.70 Billion |
| Top Companies | Amgen Inc., Novo Nordisk, Boehringer Ingelheim, Teva Pharmaceuticals |
| Last Modified Date | 31 January 2026 |
Peptide Therapeutics Market Overview
Customize Peptide Therapeutics Market Report market research report
- ✔ Get in-depth analysis of Peptide Therapeutics market size, growth, and forecasts.
- ✔ Understand Peptide Therapeutics's regional dynamics and industry-specific trends.
- ✔ Identify potential applications, end-user demand, and growth segments in Peptide Therapeutics
What is the Market Size & CAGR of Peptide Therapeutics market in 2023?
Peptide Therapeutics Industry Analysis
Peptide Therapeutics Market Segmentation and Scope
Tell us your focus area and get a customized research report.
Peptide Therapeutics Market Analysis Report by Region
Europe Peptide Therapeutics Market Report:
Europe stands as a significant player with an estimated market of $3.37 billion in 2023, projected to grow to $6 billion by 2033. The growing aging population and increased emphasis on advanced therapeutics are contributing factors.Asia Pacific Peptide Therapeutics Market Report:
The Asia Pacific region, with a market size of approximately $2 billion in 2023, is projected to reach $3.56 billion by 2033. This growth is primarily attributed to increasing investments in biotechnology and expanding healthcare infrastructures across countries like China and India.North America Peptide Therapeutics Market Report:
North America shows a robust market size of $3.56 billion in 2023, anticipated to increase to $6.34 billion by 2033. This region's growth is driven by high R&D investments and a strong focus on innovative drug development.South America Peptide Therapeutics Market Report:
In South America, the Peptide Therapeutics market is estimated to grow from $0.53 billion in 2023 to $0.94 billion by 2033. The growth is attributed to heightened healthcare spending and the expansion of research facilities focused on peptide therapeutics.Middle East & Africa Peptide Therapeutics Market Report:
The Middle East and Africa are also witnessing growth, with market sizes estimated at $1.05 billion in 2023, expected to reach $1.87 billion by 2033. Economic growth and improvements in healthcare access are key drivers in this region.Tell us your focus area and get a customized research report.
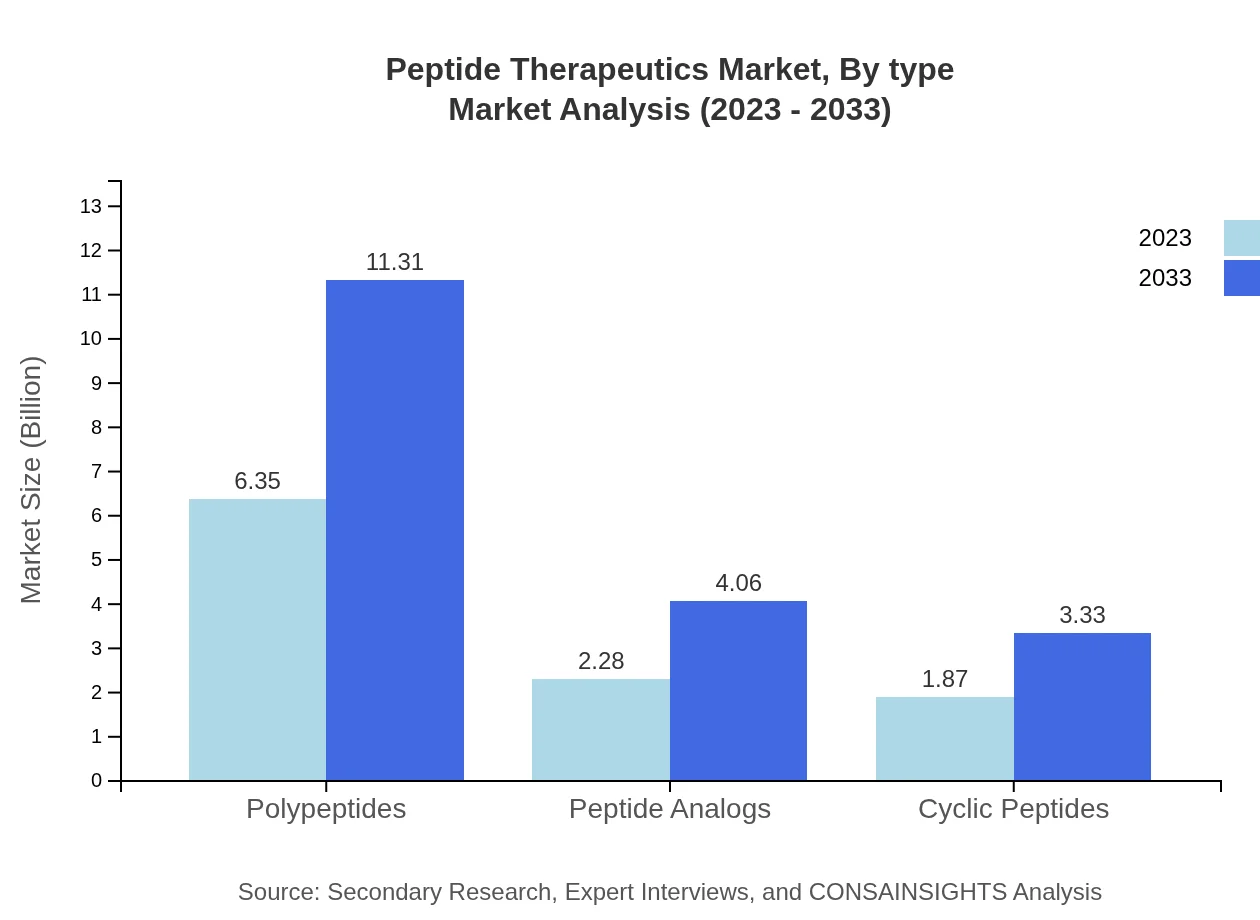
Peptide Therapeutics Market Analysis By Type
Polypeptides are leading with a market size of $6.35 billion in 2023, expected to rise to $11.31 billion by 2033, holding a market share of 60.45%. Peptide Analogs are projected to grow from $2.28 billion to $4.06 billion, marking a share of 21.73%. Cyclic Peptides will see an increase from $1.87 billion to $3.33 billion, maintaining a 17.82% share.
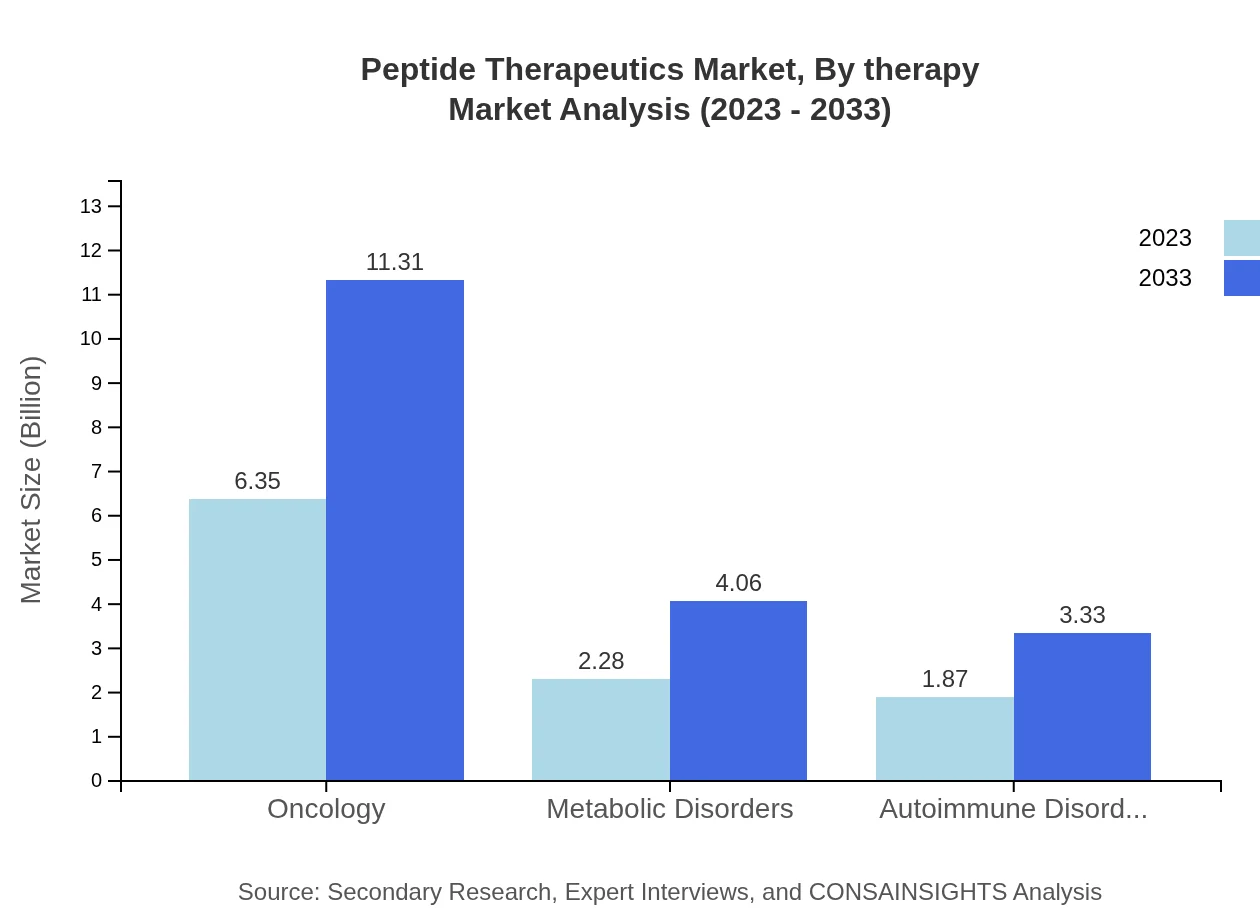
Peptide Therapeutics Market Analysis By Therapy
Oncology is a key therapy area representing a market size of $6.35 billion in 2023, growing to $11.31 billion by 2033, with a share of 60.45%. Metabolic Disorders will expand from $2.28 billion to $4.06 billion, capturing 21.73% of the market. Autoimmune Disorders will see growth from $1.87 billion to $3.33 billion with a 17.82% share.
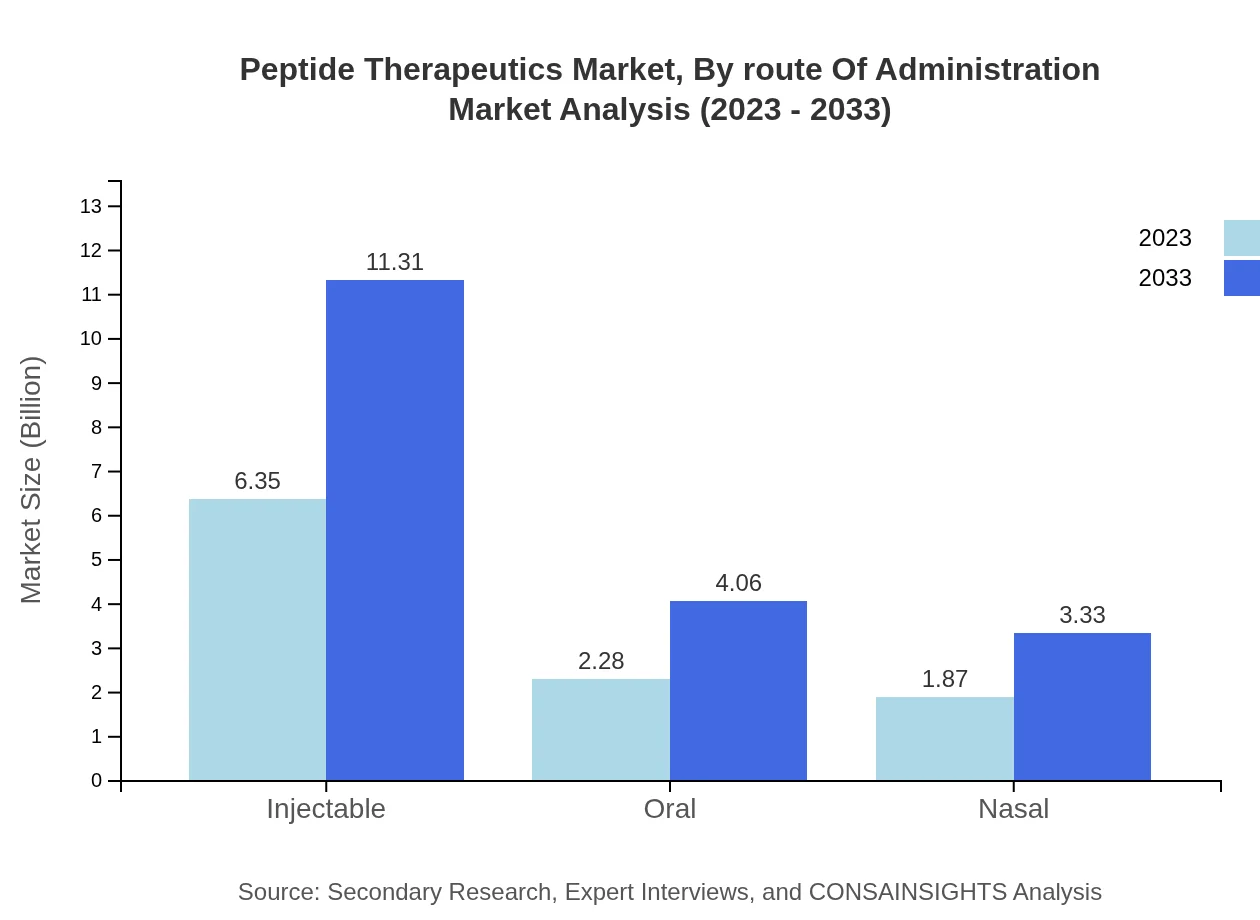
Peptide Therapeutics Market Analysis By Route Of Administration
Injectable routes dominate the market, projected to grow from $6.35 billion to $11.31 billion by 2033, maintaining a share of 60.45%. Oral administration will see growth from $2.28 billion to $4.06 billion, accounting for 21.73%. Nasal routes will expand from $1.87 billion to $3.33 billion, representing a 17.82% share.
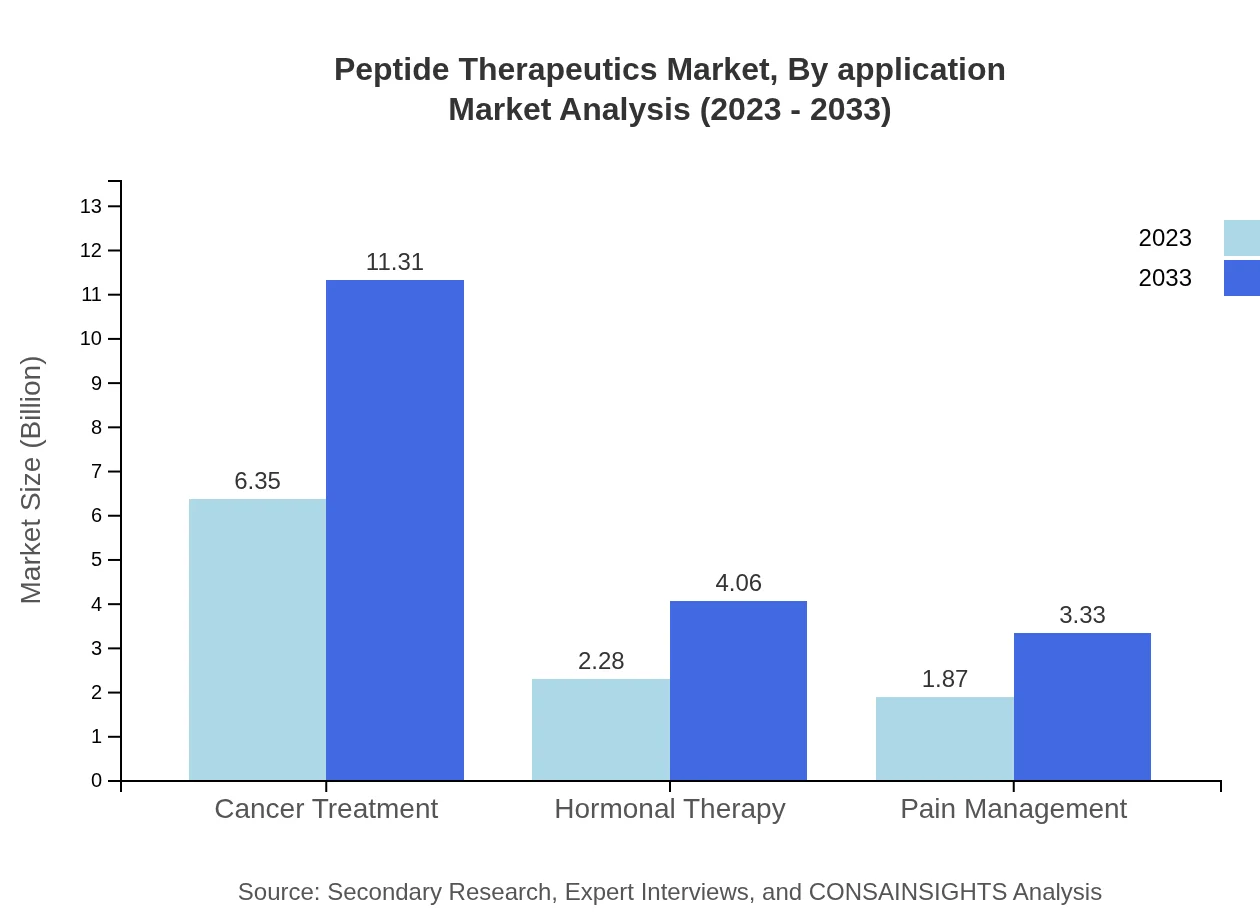
Peptide Therapeutics Market Analysis By Application
Cancer Treatment leads the application segment growing from $6.35 billion to $11.31 billion in market size, holding a 60.45% market share. Hormonal Therapy is projected to increase from $2.28 billion to $4.06 billion, accounting for 21.73%. Pain Management will grow from $1.87 billion to $3.33 billion, representing a share of 17.82%.
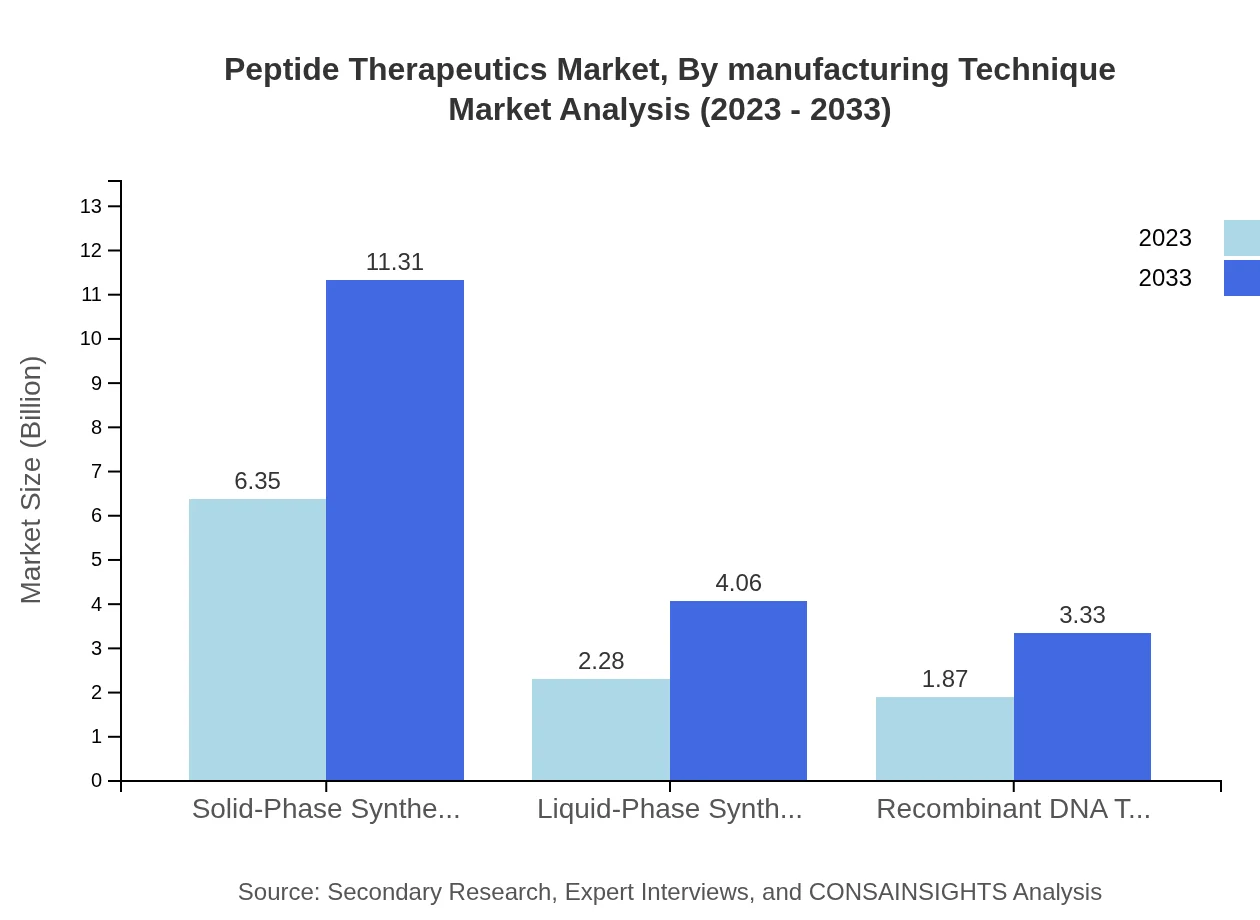
Peptide Therapeutics Market Analysis By Manufacturing Technique
Solid-Phase Synthesis is the leading manufacturing technique with a market size rising from $6.35 billion to $11.31 billion, capturing over 60.45% of the market. Liquid-Phase Synthesis is expected to grow from $2.28 billion to $4.06 billion, representing a 21.73% share. Recombinant DNA techniques will expand from $1.87 billion to $3.33 billion, accounting for 17.82%.
Peptide Therapeutics Market Trends and Future Forecast
Tell us your focus area and get a customized research report.
Global Market Leaders and Top Companies in Peptide Therapeutics Industry
Amgen Inc.:
A biotechnology company that develops innovative therapies involving peptides for oncology and metabolic conditions.Novo Nordisk:
Specializes in diabetes management products, utilizing peptide technology to create effective therapeutic solutions.Boehringer Ingelheim:
Engaged in developing peptide therapeutics for various diseases, emphasizing innovative delivery systems.Teva Pharmaceuticals:
A global leader in generic pharmaceuticals, investing in peptide therapies to expand product offerings.We're grateful to work with incredible clients.









FAQs
What is the market size of peptide Therapeutics?
The global peptide therapeutics market is estimated at USD 10.5 billion in 2023, with an expected CAGR of 5.8% from 2023 to 2033, indicating robust growth and increasing adoption of these therapies in healthcare.
What are the key market players or companies in the peptide Therapeutics industry?
Key players in the peptide therapeutics market include Eli Lilly, Amgen, Novo Nordisk, Sanofi, and Teva Pharmaceuticals. These companies are leading the development of innovative peptide-based drugs and therapies, contributing significantly to market growth.
What are the primary factors driving the growth in the peptide Therapeutics industry?
Factors driving growth include rising prevalence of chronic diseases, increasing investment in peptide drug development, advancements in peptide synthesis technologies, and the growing acceptance of peptide therapies in therapeutic areas like oncology and metabolic disorders.
Which region is the fastest Growing in the peptide Therapeutics market?
The fastest-growing region for peptide therapeutics is Europe, projected to grow from USD 3.37 billion in 2023 to USD 6.00 billion by 2033. This growth is fueled by increasing research activities and the presence of major pharmaceutical companies.
Does ConsaInsights provide customized market report data for the peptide Therapeutics industry?
Yes, ConsaInsights provides customized market reports tailored to specific needs in the peptide therapeutics industry, offering insights into market trends, growth opportunities, and competitive landscape to help businesses strategize effectively.
What deliverables can I expect from this peptide Therapeutics market research project?
Deliverables from the peptide therapeutics market research project typically include comprehensive market analysis reports, forecasts, competitive landscape assessments, segment-specific insights, and actionable recommendations for strategic planning.
What are the market trends of peptide Therapeutics?
Current trends in peptide therapeutics include innovation in peptide synthesis methods, increasing focus on targeted therapies, expansion into new therapeutic areas, and the shift towards personalized medicine, reflecting a growing market demand.