Percutaneous Transluminal Coronary Angioplasty Ptca Balloon Catheters Market Report
Published Date: 31 January 2026 | Report Code: percutaneous-transluminal-coronary-angioplasty-ptca-balloon-catheters
Percutaneous Transluminal Coronary Angioplasty Ptca Balloon Catheters Market Size, Share, Industry Trends and Forecast to 2033
This report delivers a comprehensive analysis of the Percutaneous Transluminal Coronary Angioplasty (PTCA) Balloon Catheters market, covering key insights, trends, and forecasts from 2023 to 2033.
| Metric | Value |
|---|---|
| Study Period | 2023 - 2033 |
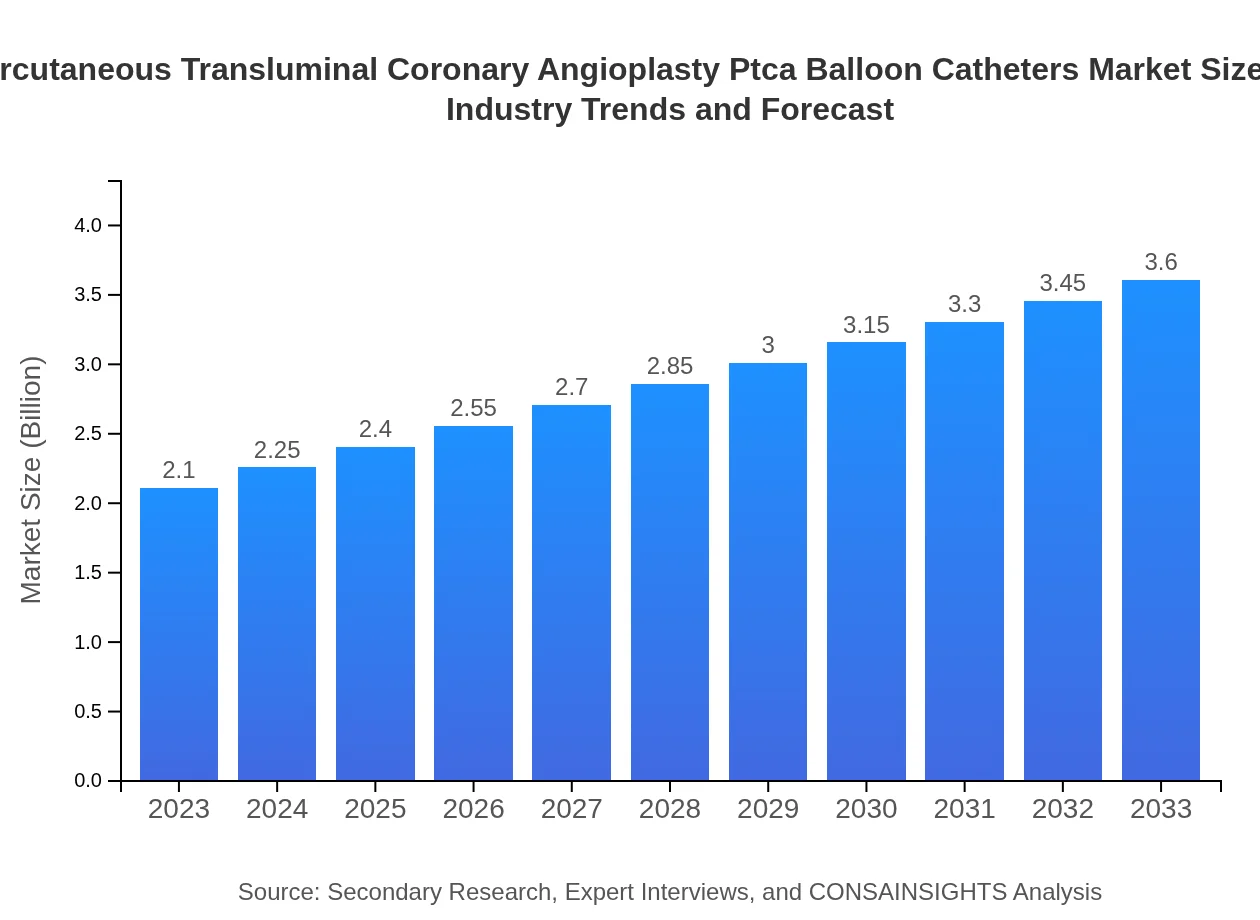
| 2023 Market Size | $2.10 Billion |
| CAGR (2023-2033) | 5.4% |
| 2033 Market Size | $3.60 Billion |
| Top Companies | Abbott Laboratories, Boston Scientific Corporation, Medtronic PLC, B. Braun Melsungen AG |
| Last Modified Date | 31 January 2026 |
Percutaneous Transluminal Coronary Angioplasty Ptca Balloon Catheters Market Overview
Customize Percutaneous Transluminal Coronary Angioplasty Ptca Balloon Catheters Market Report market research report
- ✔ Get in-depth analysis of Percutaneous Transluminal Coronary Angioplasty Ptca Balloon Catheters market size, growth, and forecasts.
- ✔ Understand Percutaneous Transluminal Coronary Angioplasty Ptca Balloon Catheters's regional dynamics and industry-specific trends.
- ✔ Identify potential applications, end-user demand, and growth segments in Percutaneous Transluminal Coronary Angioplasty Ptca Balloon Catheters
What is the Market Size & CAGR of Percutaneous Transluminal Coronary Angioplasty Ptca Balloon Catheters market in 2023?
Percutaneous Transluminal Coronary Angioplasty Ptca Balloon Catheters Industry Analysis
Percutaneous Transluminal Coronary Angioplasty Ptca Balloon Catheters Market Segmentation and Scope
Tell us your focus area and get a customized research report.
Percutaneous Transluminal Coronary Angioplasty Ptca Balloon Catheters Market Analysis Report by Region
Europe Percutaneous Transluminal Coronary Angioplasty Ptca Balloon Catheters Market Report:
Europe shows a robust market for PTCA balloon catheters, growing from $0.65 billion in 2023 to $1.11 billion by 2033. The region's emphasis on quality healthcare, coupled with aging population demographics, supports this expansion.Asia Pacific Percutaneous Transluminal Coronary Angioplasty Ptca Balloon Catheters Market Report:
The Asia Pacific region is witnessing substantial growth, projected to expand from $0.4 billion in 2023 to $0.68 billion by 2033. Rising healthcare spending and increasing awareness about cardiovascular health aid in this growth. Countries like India and China are significantly investing in advanced healthcare technologies.North America Percutaneous Transluminal Coronary Angioplasty Ptca Balloon Catheters Market Report:
The North American market is the largest, projected to grow from $0.74 billion in 2023 to $1.26 billion by 2033. High rates of coronary interventions, strong reimbursement policies, and advanced healthcare facilities contribute to this growth.South America Percutaneous Transluminal Coronary Angioplasty Ptca Balloon Catheters Market Report:
In South America, the market is expected to increase from $0.19 billion in 2023 to $0.33 billion by 2033. Growth in this region is mainly driven by increasing prevalence of heart diseases and expanding healthcare access.Middle East & Africa Percutaneous Transluminal Coronary Angioplasty Ptca Balloon Catheters Market Report:
In the Middle East and Africa, the market is expected to grow moderately from $0.12 billion in 2023 to $0.21 billion by 2033. Healthcare infrastructure development and increased availability of medical devices are pivotal contributors.Tell us your focus area and get a customized research report.
Percutaneous Transluminal Coronary Angioplasty Ptca Balloon Catheters Market Analysis By Product Type
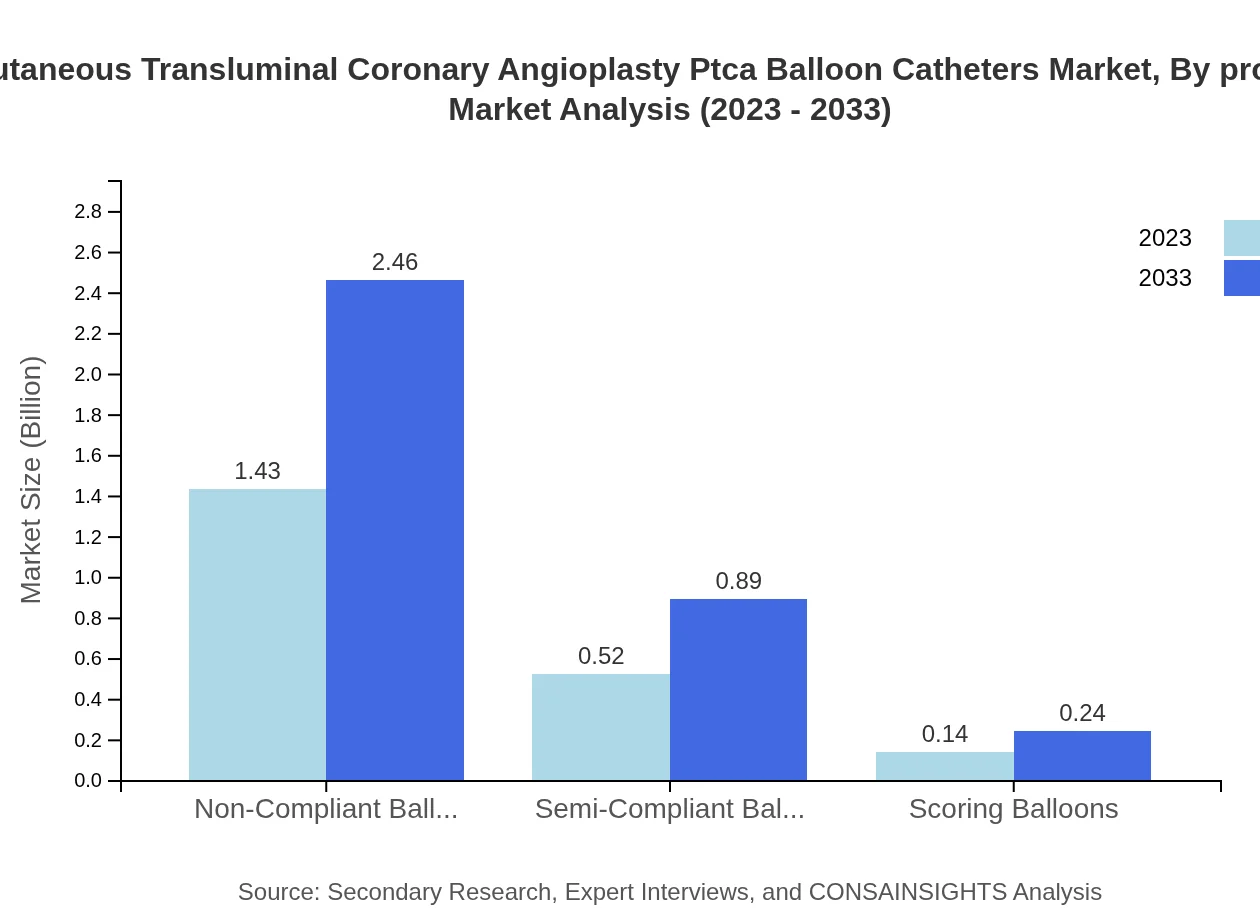
In this segment, non-compliant balloons dominate the PTCA market due to their reliability in high-pressure scenarios, holding a market size of $1.43 billion in 2023 and expected to increase to $2.46 billion by 2033. Semi-compliant and scoring balloons follow, offering unique advantages for specific cases.
Percutaneous Transluminal Coronary Angioplasty Ptca Balloon Catheters Market Analysis By End User
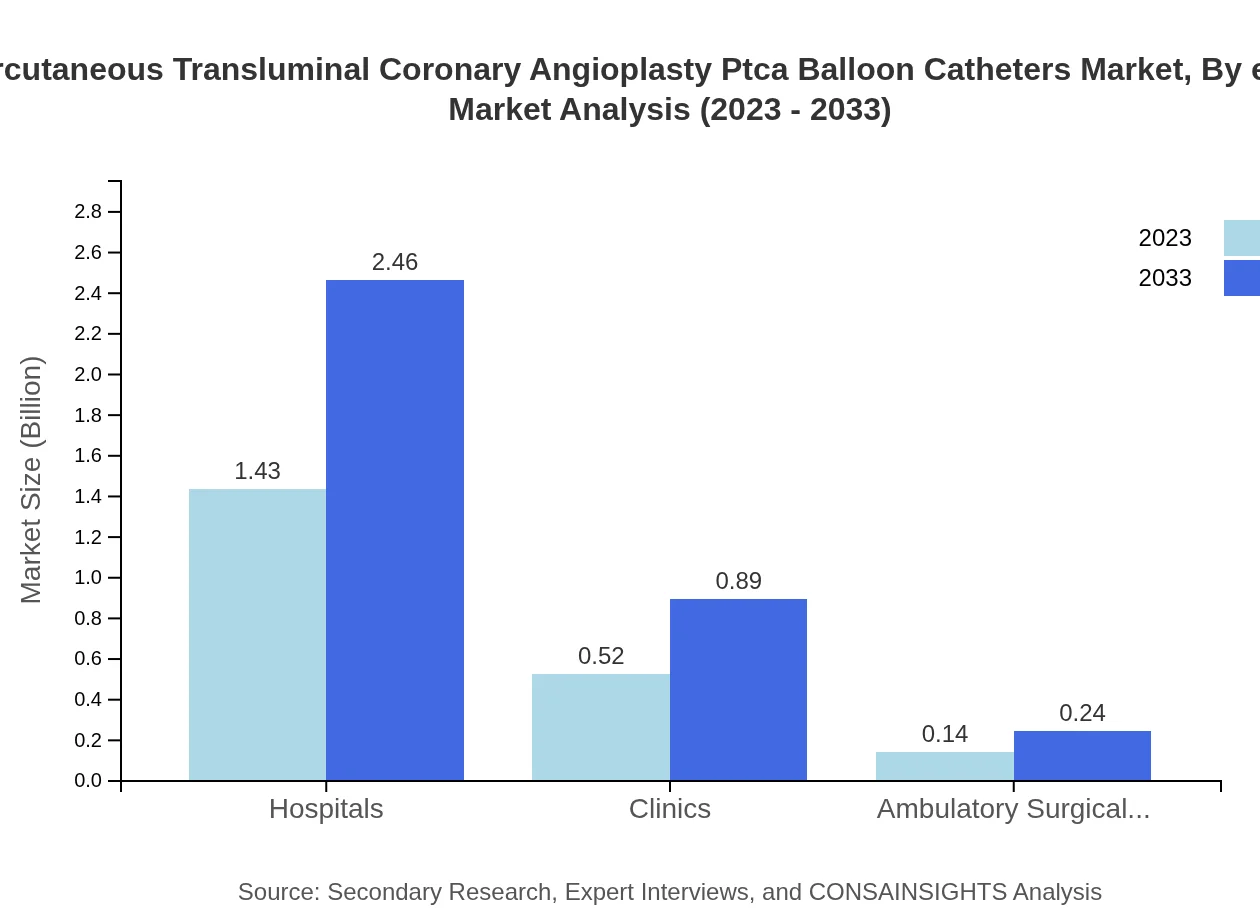
Hospitals represent the leading end-user segment with a market size of $1.43 billion in 2023, reflecting constant demand for advanced cardiac interventional devices, projected to rise to $2.46 billion by 2033. Clinics and ambulatory surgical centers occupy smaller but significant shares.
Percutaneous Transluminal Coronary Angioplasty Ptca Balloon Catheters Market Analysis By Application
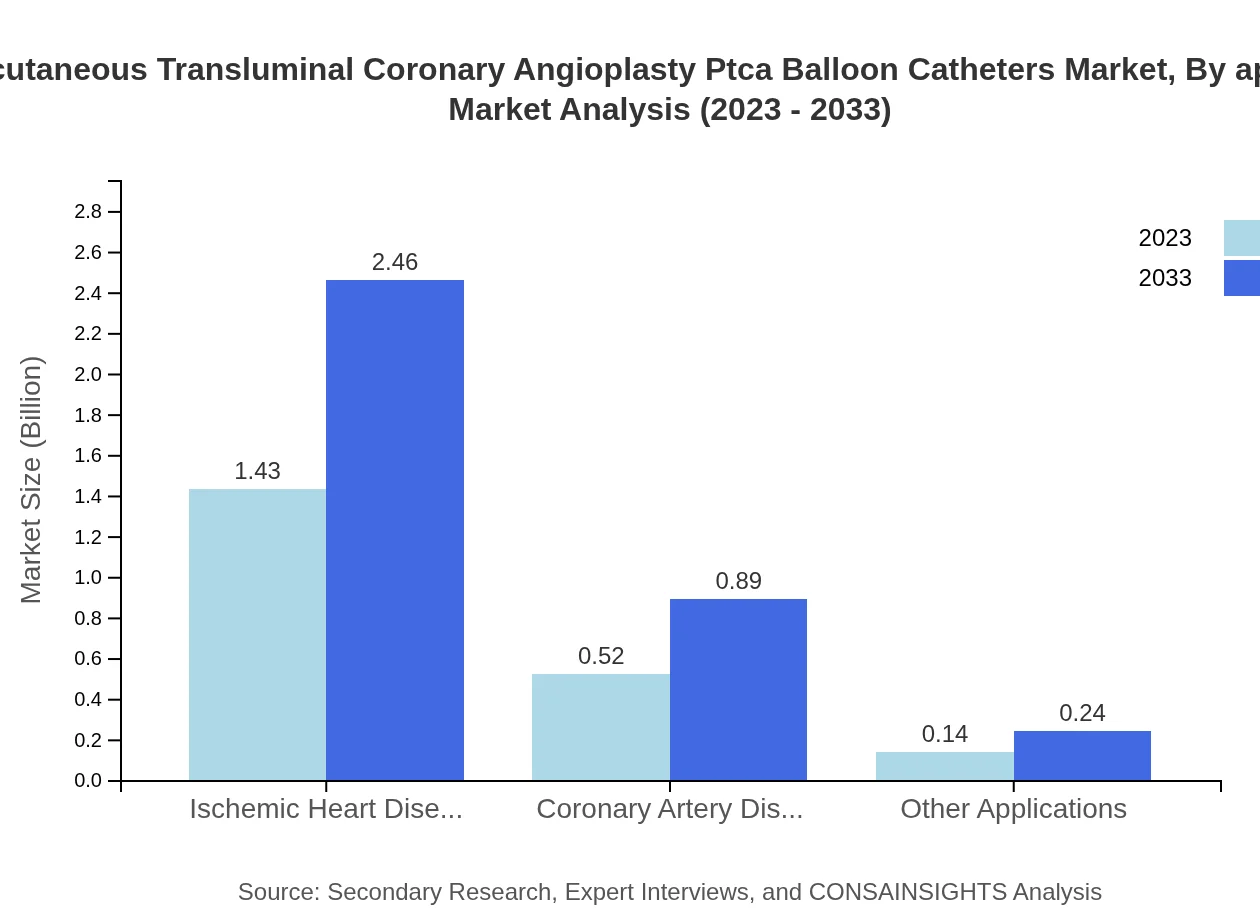
The majority of applications focus on ischemic heart disease, holding a market value of $1.43 billion in 2023 expected to reach $2.46 billion by 2033. This segment is crucial due to the increasing incidence of coronary artery disease and effective treatment demand.
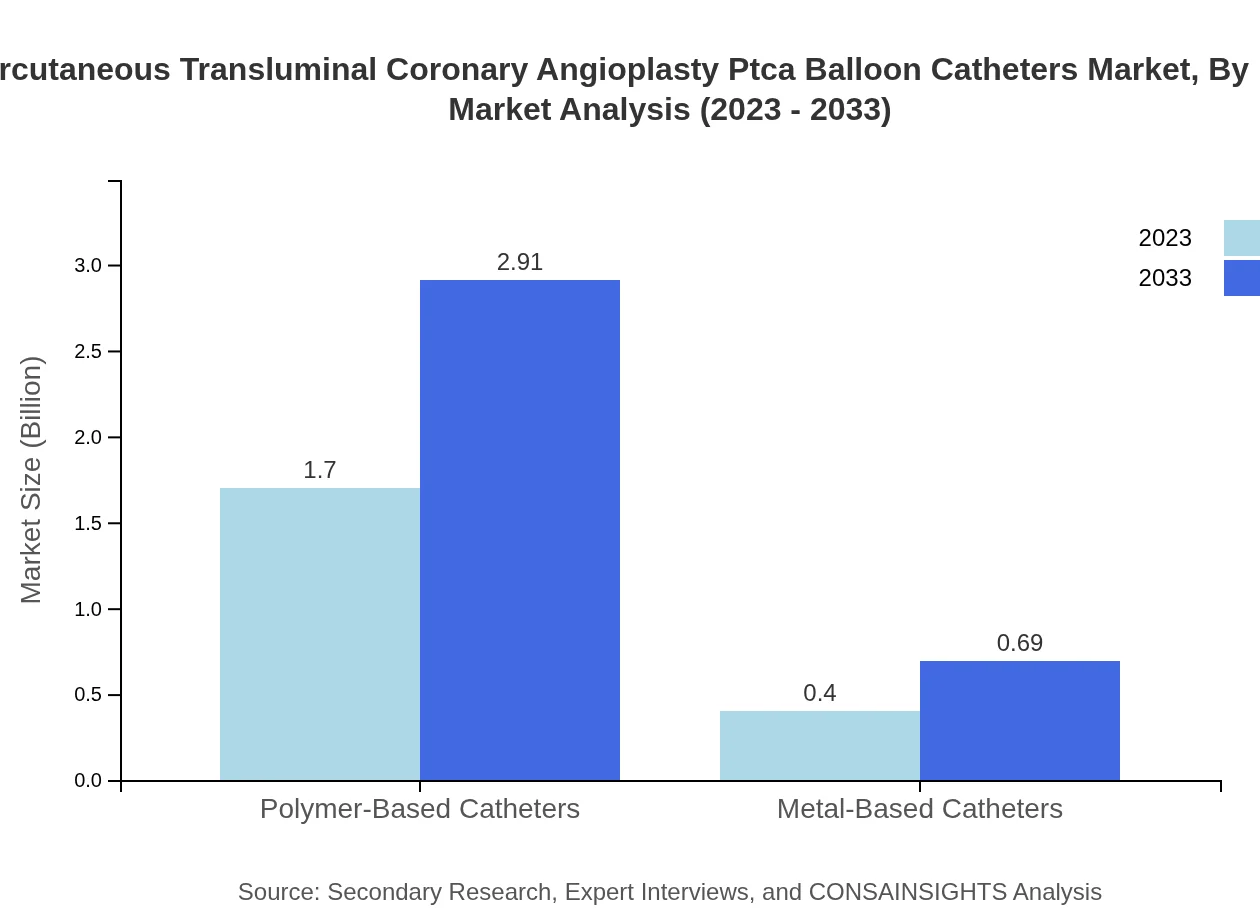
Percutaneous Transluminal Coronary Angioplasty Ptca Balloon Catheters Market Analysis By Material
Polymer-based catheters represent the highest market share of approximately $1.70 billion in 2023, growing to $2.91 billion by 2033. Their superior flexibility and safety features are driving their preference over metal-based options.
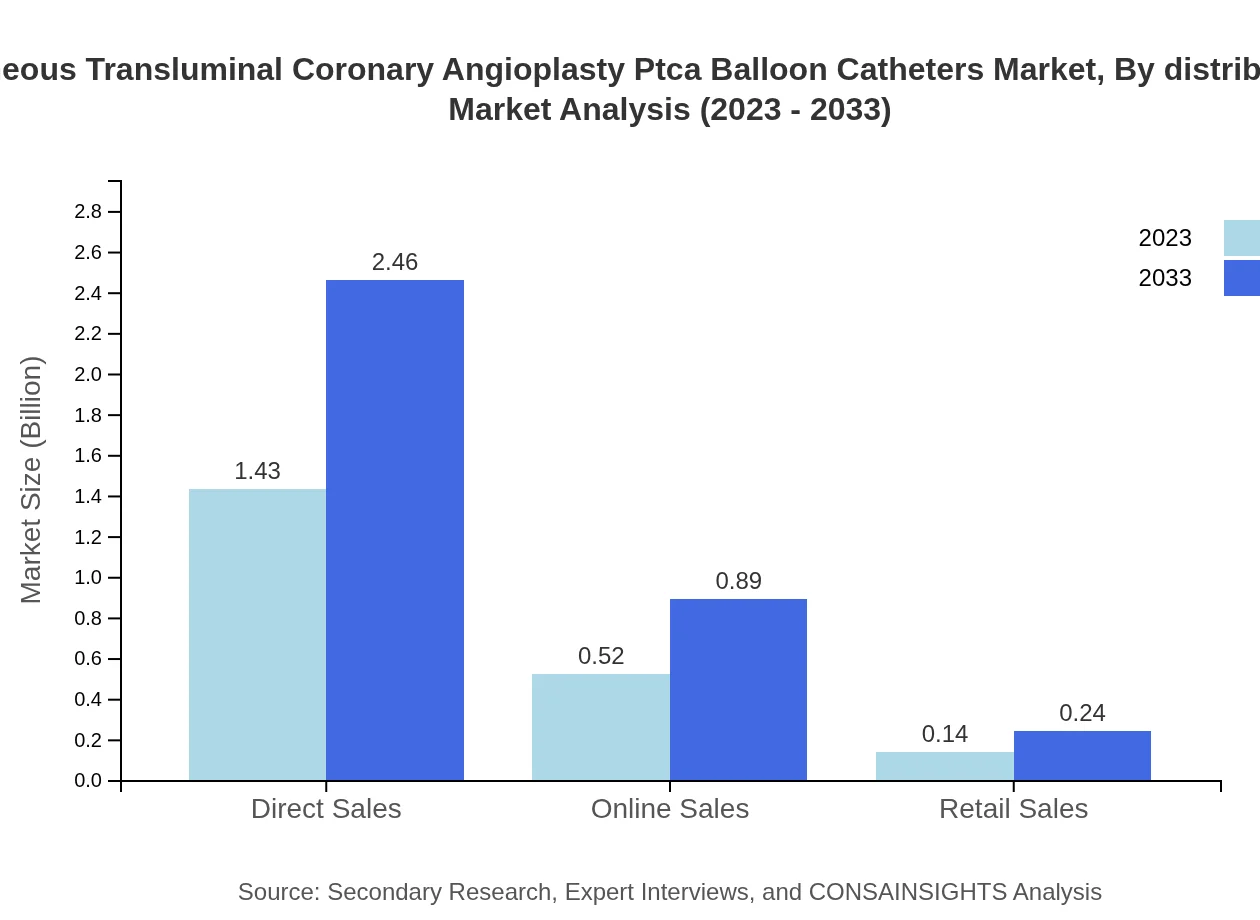
Percutaneous Transluminal Coronary Angioplasty Ptca Balloon Catheters Market Analysis By Distribution Channel
Direct sales hold a dominant market share of $1.43 billion in 2023, while online sales are rapidly capturing attention, growing from $0.52 billion to $0.89 billion by 2033, thanks to increased online purchasing and e-health initiatives.
Percutaneous Transluminal Coronary Angioplasty Ptca Balloon Catheters Market Trends and Future Forecast
Tell us your focus area and get a customized research report.
Global Market Leaders and Top Companies in Percutaneous Transluminal Coronary Angioplasty Ptca Balloon Catheters Industry
Abbott Laboratories:
Known for its advanced medical devices, Abbott leads the market with innovations such as drug-eluting balloon catheters for advanced cardiovascular treatments.Boston Scientific Corporation:
A key player in the medical device sector, Boston Scientific offers a wide range of PTCA balloon catheters featuring patented technologies for superior performance.Medtronic PLC:
With a focus on improving patient outcomes, Medtronic has established itself as a leader in cardiovascular products, including various PTCA balloon catheters.B. Braun Melsungen AG:
B. Braun specializes in healthcare products and solutions, including innovative PTCA devices designed to enhance procedural efficacy.We're grateful to work with incredible clients.









FAQs
What is the market size of percutaneous Transluminal Coronary Angioplasty Ptca Balloon Catheters?
The global percutaneous transluminal coronary angioplasty (PTCA) balloon catheters market was valued at approximately $2.1 billion in 2023, with a projected CAGR of 5.4% through 2033, showing significant growth potential.
What are the key market players or companies in this percutaneous Transluminal Coronary Angioplasty Ptca Balloon Catheters industry?
Key market players in the PTCA balloon catheters industry include major medical device manufacturers known for cardiovascular products. These companies focus on innovation and strategic partnerships to enhance their market presence.
What are the primary factors driving the growth in the percutaneous Transluminal Coronary Angioplasty Ptca Balloon Catheters industry?
Growth factors include the rising prevalence of coronary artery diseases, advancements in catheter technology, and increasing healthcare expenditure. The aging population also contributes to heightened demand for effective cardiac procedures.
Which region is the fastest Growing in the percutaneous Transluminal Coronary Angioplasty Ptca Balloon Catheters?
The Asia Pacific region shows rapid growth in the PTCA balloon catheters market, projected to expand from $0.40 billion in 2023 to $0.68 billion by 2033, driven by improving healthcare infrastructure.
Does ConsaInsights provide customized market report data for the percutaneous Transluminal Coronary Angioplasty Ptca Balloon Catheters industry?
Yes, ConsaInsights offers customized market report data tailored to meet specific client needs, providing in-depth insights and detailed analysis into the PTCA balloon catheters market.
What deliverables can I expect from this percutaneous Transluminal Coronary Angioplasty Ptca Balloon Catheters market research project?
Deliverables include a comprehensive market report, detailed analysis of trends, competitive landscape, forecasts, and insights into regional and segment-specific growth for PTCA balloon catheters.
What are the market trends of percutaneous Transluminal Coronary Angioplasty Ptca Balloon Catheters?
Trends include a shift towards minimally invasive procedures, increasing adoption of advanced catheter technologies, and a focus on polymer-based catheters, predicted to dominate the market share by 80.83%.