Pertussis Vaccine Market Report
Published Date: 31 January 2026 | Report Code: pertussis-vaccine
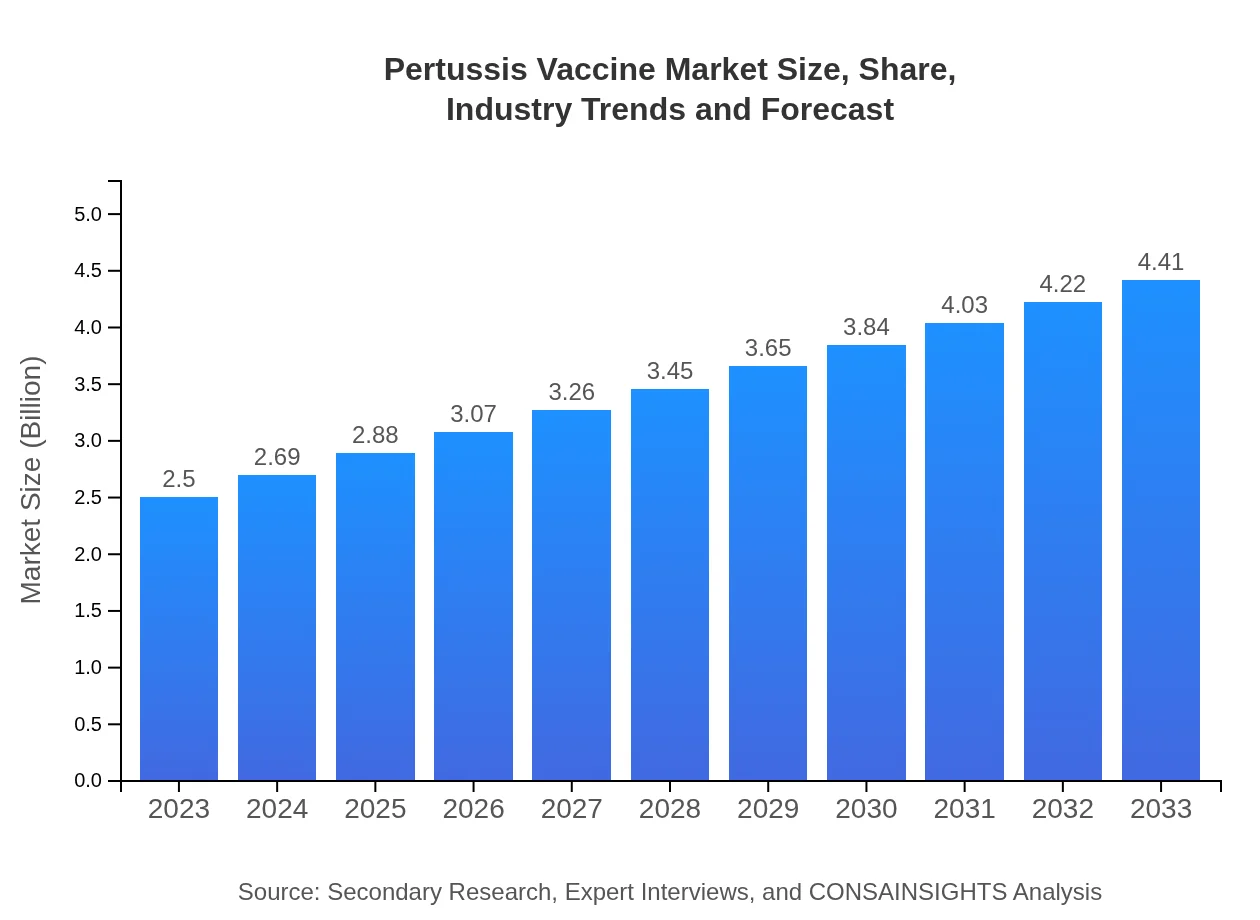
Pertussis Vaccine Market Size, Share, Industry Trends and Forecast to 2033
This market report provides an in-depth analysis of the Pertussis Vaccine market from 2023 to 2033, highlighting market size, growth trends, industry dynamics, and regional insights.
| Metric | Value |
|---|---|
| Study Period | 2023 - 2033 |
| 2023 Market Size | $2.50 Billion |
| CAGR (2023-2033) | 5.7% |
| 2033 Market Size | $4.41 Billion |
| Top Companies | Sanofi Pasteur, GlaxoSmithKline, Merck & Co., Pfizer |
| Last Modified Date | 31 January 2026 |
Pertussis Vaccine Market Overview
Customize Pertussis Vaccine Market Report market research report
- ✔ Get in-depth analysis of Pertussis Vaccine market size, growth, and forecasts.
- ✔ Understand Pertussis Vaccine's regional dynamics and industry-specific trends.
- ✔ Identify potential applications, end-user demand, and growth segments in Pertussis Vaccine
What is the Market Size & CAGR of Pertussis Vaccine market in 2023?
Pertussis Vaccine Industry Analysis
Pertussis Vaccine Market Segmentation and Scope
Tell us your focus area and get a customized research report.
Pertussis Vaccine Market Analysis Report by Region
Europe Pertussis Vaccine Market Report:
Europe represents a mature market for Pertussis Vaccines, with values moving from $0.69 billion in 2023 to $1.21 billion by 2033. Heightened awareness of vaccine-preventable diseases and robust vaccination programs underpin the steady growth in the region.Asia Pacific Pertussis Vaccine Market Report:
The Asia Pacific region, valued at $0.52 billion in 2023 and projected to reach $0.91 billion by 2033, is witnessing accelerated growth due to rising birth rates and increased government initiatives promoting vaccination. Countries like India and China play a pivotal role in the regional market.North America Pertussis Vaccine Market Report:
The North American market, valued at $0.83 billion in 2023 and projected to grow to $1.47 billion by 2033, showcases strong market innovation and accessibility. The presence of established healthcare systems and public health campaigns emphasizes the importance of pertussis vaccination.South America Pertussis Vaccine Market Report:
In South America, the Pertussis Vaccine market size is expected to grow from $0.23 billion in 2023 to $0.41 billion by 2033. The focus on childhood vaccination programs and improved healthcare infrastructure contributes to this growth.Middle East & Africa Pertussis Vaccine Market Report:
The Middle East and Africa region has a projected market size increase from $0.23 billion in 2023 to $0.41 billion in 2033. Increasing healthcare access and government initiatives focusing on immunization highlight the region's potential growth.Tell us your focus area and get a customized research report.
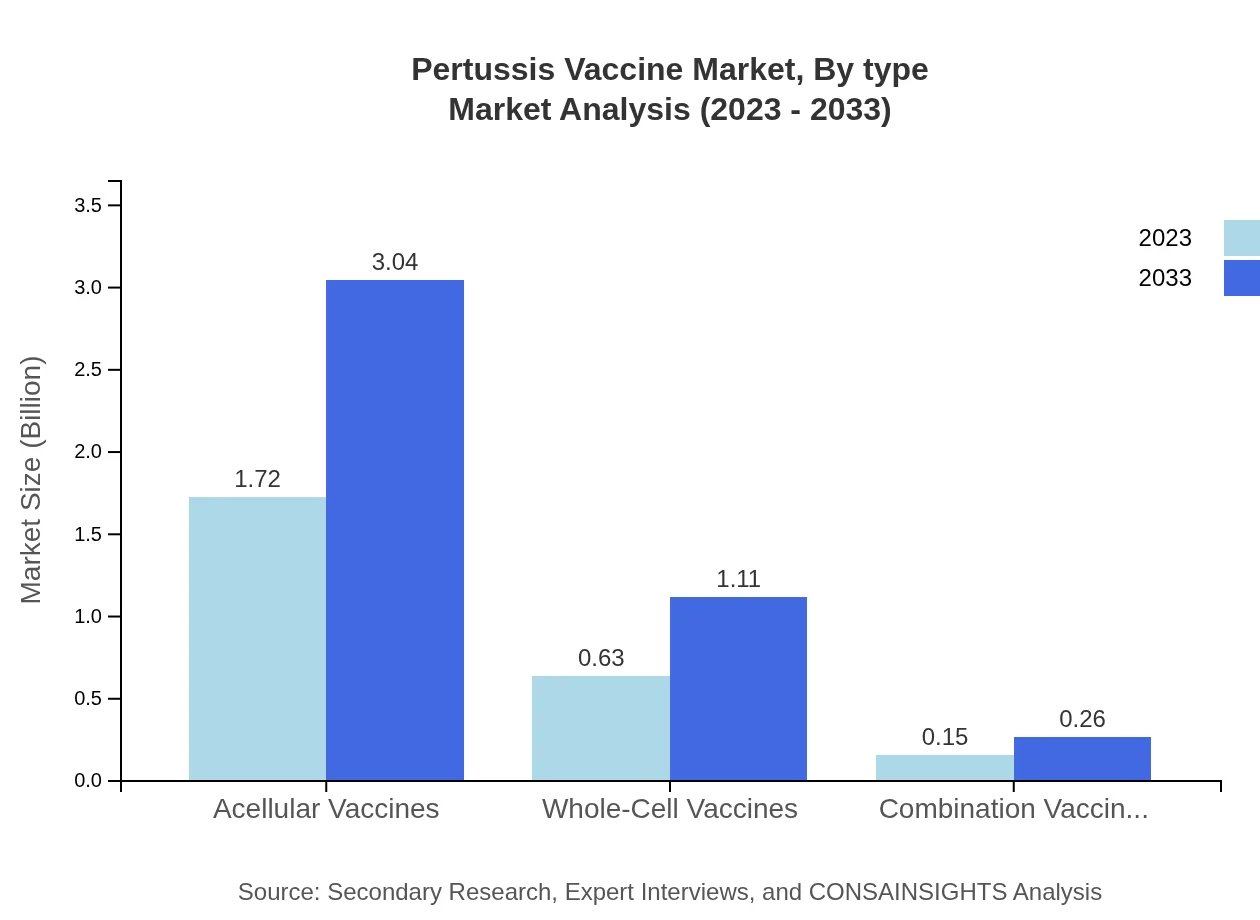
Pertussis Vaccine Market Analysis By Type
The Pertussis Vaccine market is largely driven by acellular vaccines, which are expected to rise from $1.72 billion in 2023 to $3.04 billion in 2033. Whole-cell vaccines maintain a significant presence, growing from $0.63 billion to $1.11 billion over the same period. Combination vaccines remain niche but are projected to expand slightly due to combination immunization schedules.
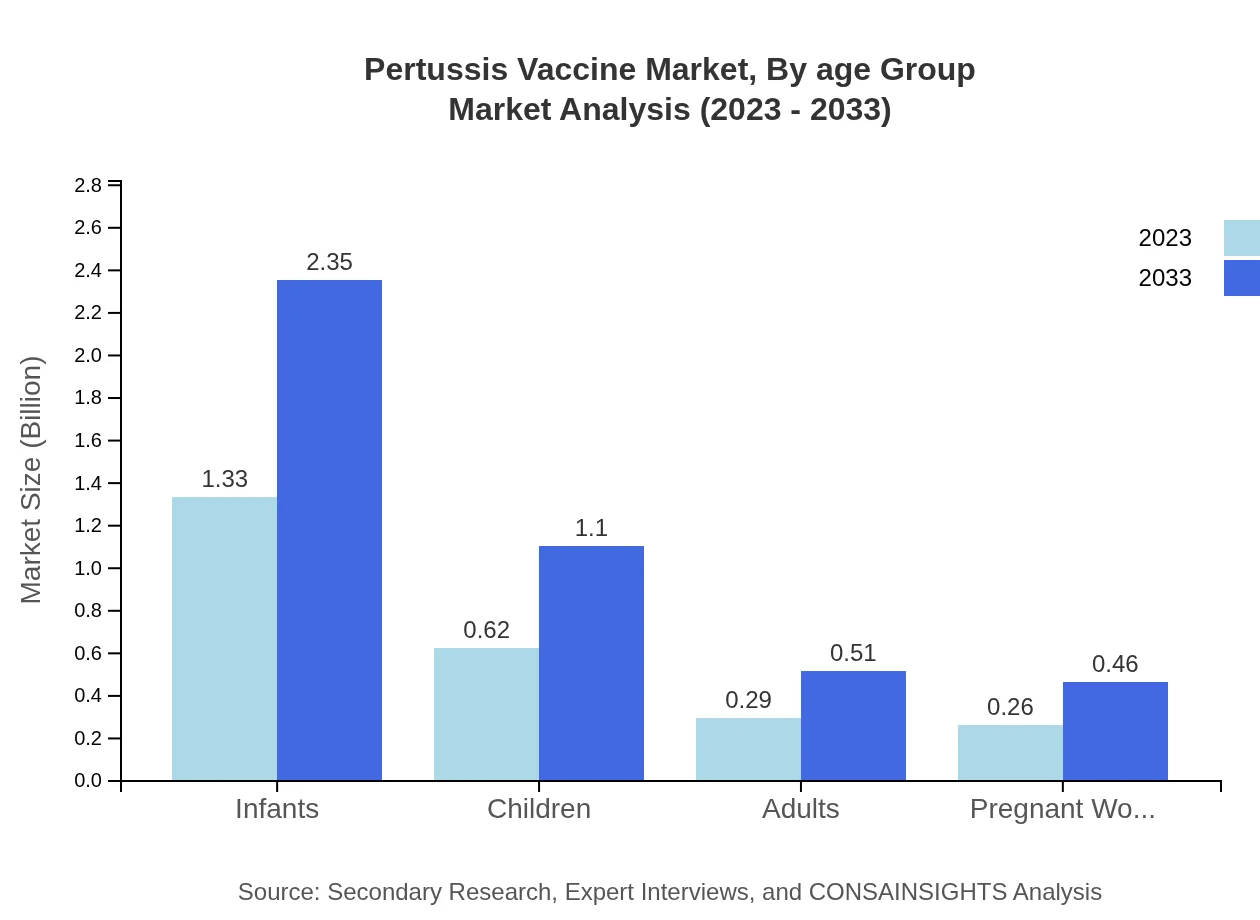
Pertussis Vaccine Market Analysis By Age Group
The largest segment in the Pertussis Vaccine market is infants, with an expected market growth from $1.33 billion in 2023 to $2.35 billion in 2033. Children and adults represent smaller segments but will see increased focus on vaccination during routine healthcare visits due to rising awareness.
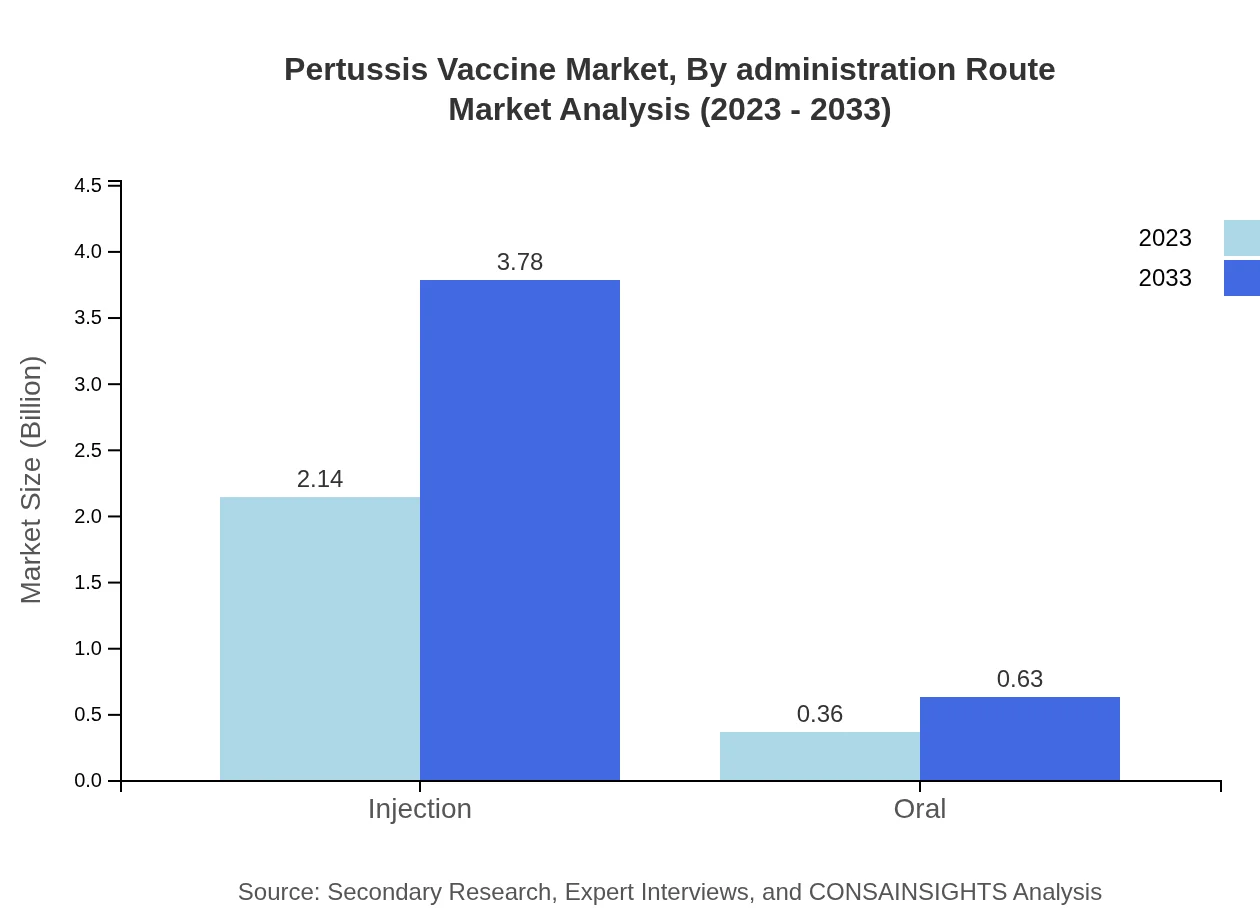
Pertussis Vaccine Market Analysis By Administration Route
Injection remains the predominant administration route for Pertussis Vaccines, growing from $2.14 billion in 2023 to $3.78 billion in 2033. Oral vaccines, while less common, are projected to see gradual adoption due to preference for non-invasive methods.
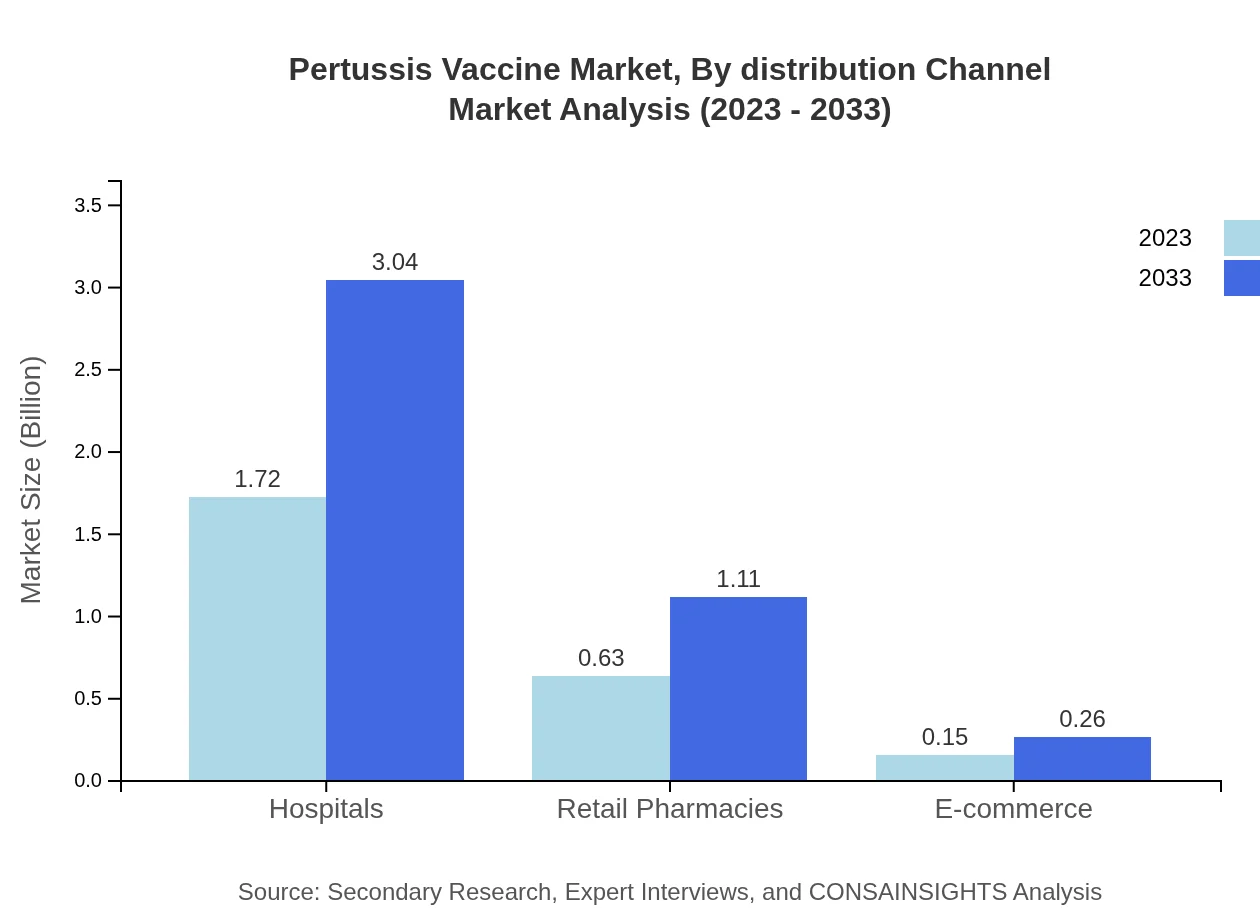
Pertussis Vaccine Market Analysis By Distribution Channel
Hospitals are the leading distribution channel for Pertussis Vaccines, valued at $1.72 billion in 2023, with expectations to expand to $3.04 billion by 2033. Retail pharmacies and e-commerce are gradually gaining traction as alternative channels, particularly in regions seeking improved accessibility.
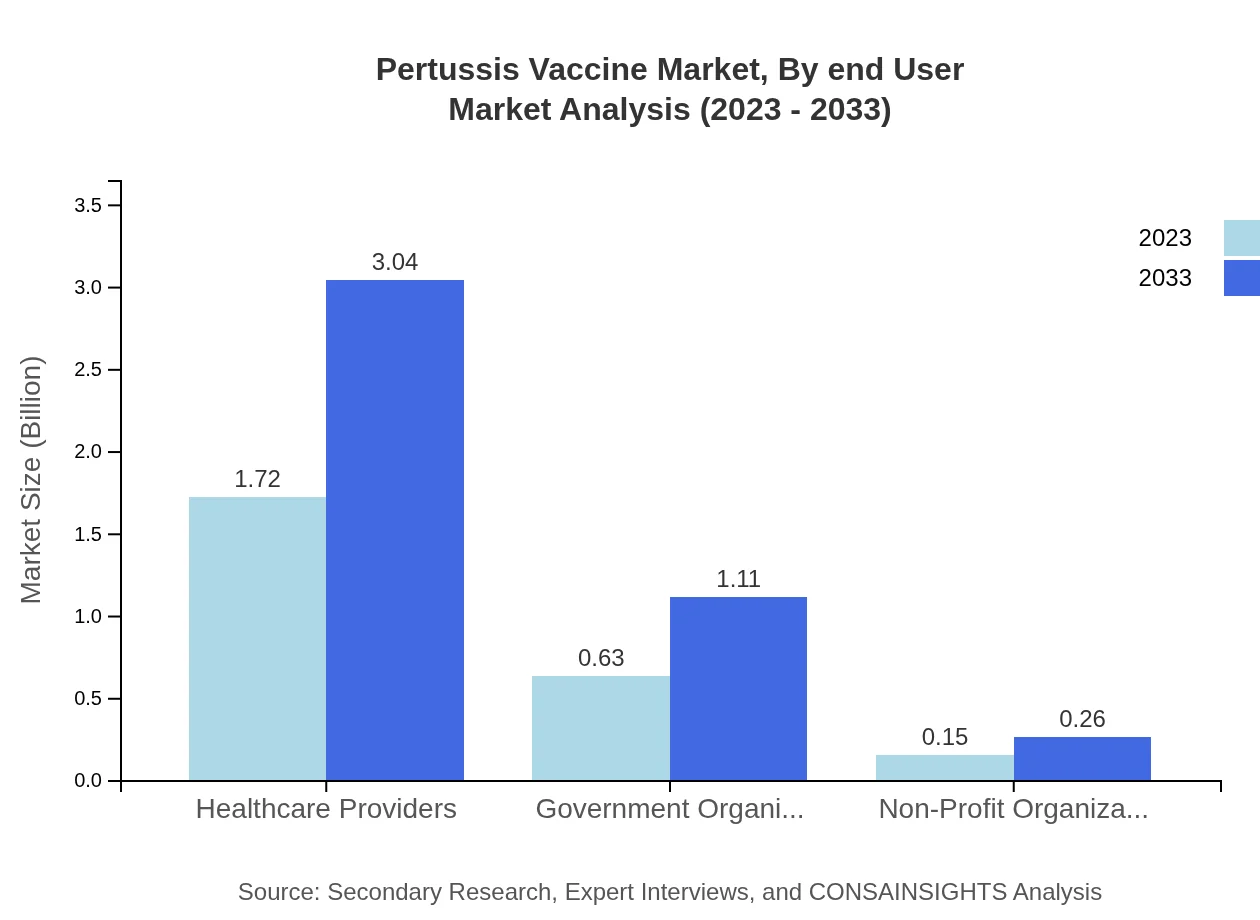
Pertussis Vaccine Market Analysis By End User
Healthcare providers dominate the end-user segment, comprising a substantial share. Government organizations and non-profit organizations also significantly contribute to the distribution and administration of the vaccine, focusing mainly on public health initiatives and childhood vaccination drives.
Pertussis Vaccine Market Trends and Future Forecast
Tell us your focus area and get a customized research report.
Global Market Leaders and Top Companies in Pertussis Vaccine Industry
Sanofi Pasteur:
A leading biotechnology firm known for developing Vaccines and biologics, significantly contributing to global immunization efforts, including the Pertussis Vaccine.GlaxoSmithKline:
A major player in the vaccine industry focused on innovative vaccine development, including combination vaccines that incorporate pertussis components.Merck & Co.:
This company plays a critical role in the global vaccine market, offering several vaccines and continuously investing in research and development of Pertussis Vaccines.Pfizer :
Pfizer contributes to the pertussis vaccine market significantly, particularly in developing new formulations for various age groups, enhancing public health outcomes.We're grateful to work with incredible clients.









FAQs
What is the market size of the pertussis vaccine?
The global pertussis vaccine market is valued at approximately $2.5 billion in 2023, with a projected growth rate of 5.7% CAGR, indicating a robust expansion and investment potential through 2033.
What are the key market players or companies in the pertussis vaccine industry?
Key players in the pertussis vaccine market include major pharmaceutical companies such as Sanofi, GlaxoSmithKline, Merck & Co., and Pfizer, which lead in vaccine research, development, and production.
What are the primary factors driving the growth in the pertussis vaccine industry?
The growth of the pertussis vaccine industry is driven by increasing prevalence of pertussis infections, government vaccination programs, rising awareness regarding vaccination benefits, and continuous advancements in vaccine technology.
Which region is the fastest Growing in the pertussis vaccine?
The fastest-growing region for the pertussis vaccine market is North America, expected to grow from $0.83 billion in 2023 to $1.47 billion by 2033, due to strong healthcare infrastructure and vaccination initiatives.
Does ConsaInsights provide customized market report data for the pertussis vaccine industry?
Yes, ConsaInsights offers customized market report data specific to the pertussis vaccine industry, tailored to meet individual client needs, including insights into market size, trends, and forecasts.
What deliverables can I expect from this pertussis vaccine market research project?
Deliverables from the pertussis vaccine market research project typically include detailed market analyses, segmentation data, competitive landscape review, trend overviews, and strategic recommendations.
What are the market trends of the pertussis vaccine?
Current trends in the pertussis vaccine market include a shift towards acellular vaccines, rising immunization rates in developing countries, and increased acceptance of combination vaccines to enhance efficacy and coverage.