Pharmaceutical Anticounterfeiting Technologies Market Report
Published Date: 31 January 2026 | Report Code: pharmaceutical-anticounterfeiting-technologies
Pharmaceutical Anticounterfeiting Technologies Market Size, Share, Industry Trends and Forecast to 2033
This report explores the Pharmaceutical Anticounterfeiting Technologies market, providing comprehensive insights, analysis from 2023 to 2033, including market sizes, growth trends, segmentation, regional insights, and future forecasts.
| Metric | Value |
|---|---|
| Study Period | 2023 - 2033 |
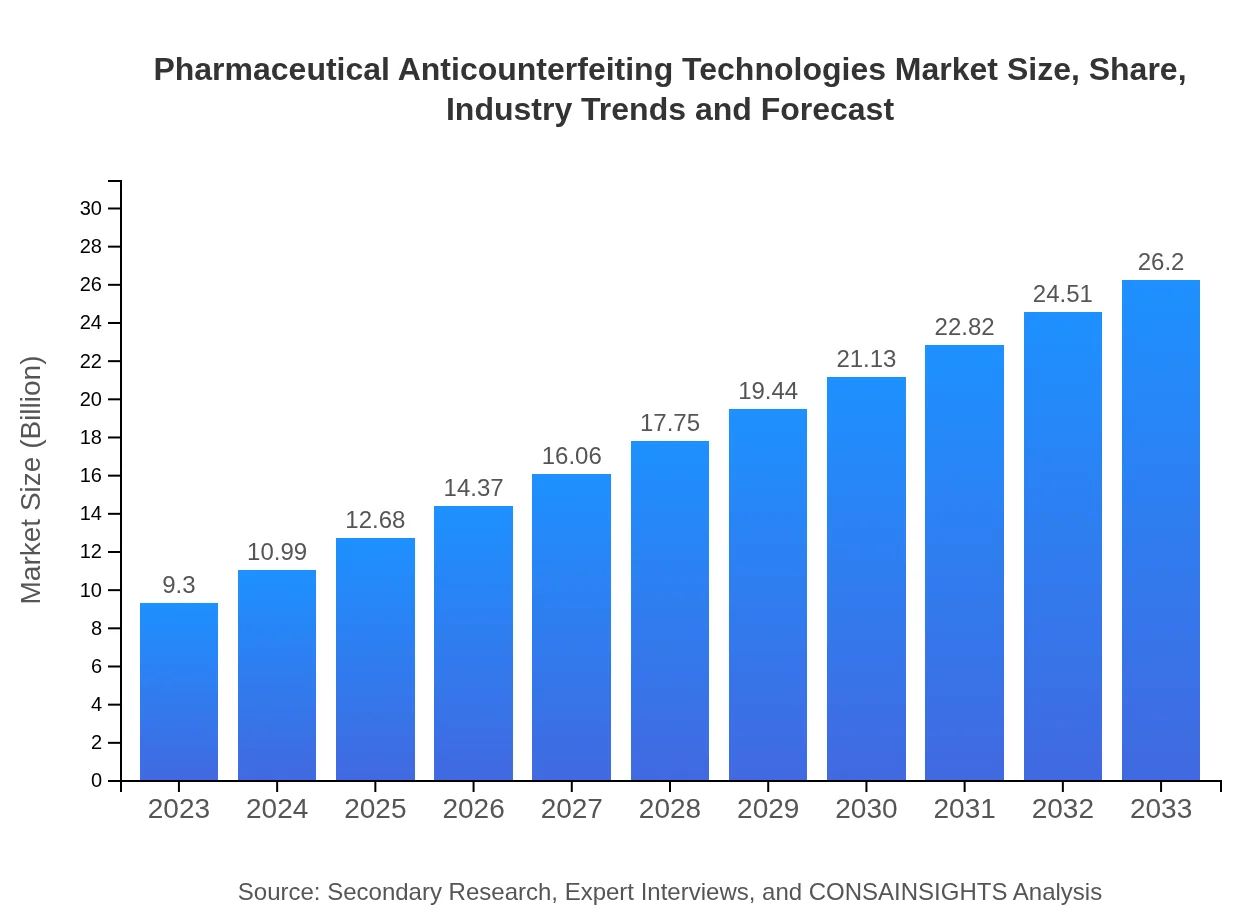
| 2023 Market Size | $9.30 Billion |
| CAGR (2023-2033) | 10.5% |
| 2033 Market Size | $26.20 Billion |
| Top Companies | Systech International, Authenta, Zebra Technologies, PharmaSecure |
| Last Modified Date | 31 January 2026 |
Pharmaceutical Anticounterfeiting Technologies Market Overview
Customize Pharmaceutical Anticounterfeiting Technologies Market Report market research report
- ✔ Get in-depth analysis of Pharmaceutical Anticounterfeiting Technologies market size, growth, and forecasts.
- ✔ Understand Pharmaceutical Anticounterfeiting Technologies's regional dynamics and industry-specific trends.
- ✔ Identify potential applications, end-user demand, and growth segments in Pharmaceutical Anticounterfeiting Technologies
What is the Market Size & CAGR of Pharmaceutical Anticounterfeiting Technologies market in 2023?
Pharmaceutical Anticounterfeiting Technologies Industry Analysis
Pharmaceutical Anticounterfeiting Technologies Market Segmentation and Scope
Tell us your focus area and get a customized research report.
Pharmaceutical Anticounterfeiting Technologies Market Analysis Report by Region
Europe Pharmaceutical Anticounterfeiting Technologies Market Report:
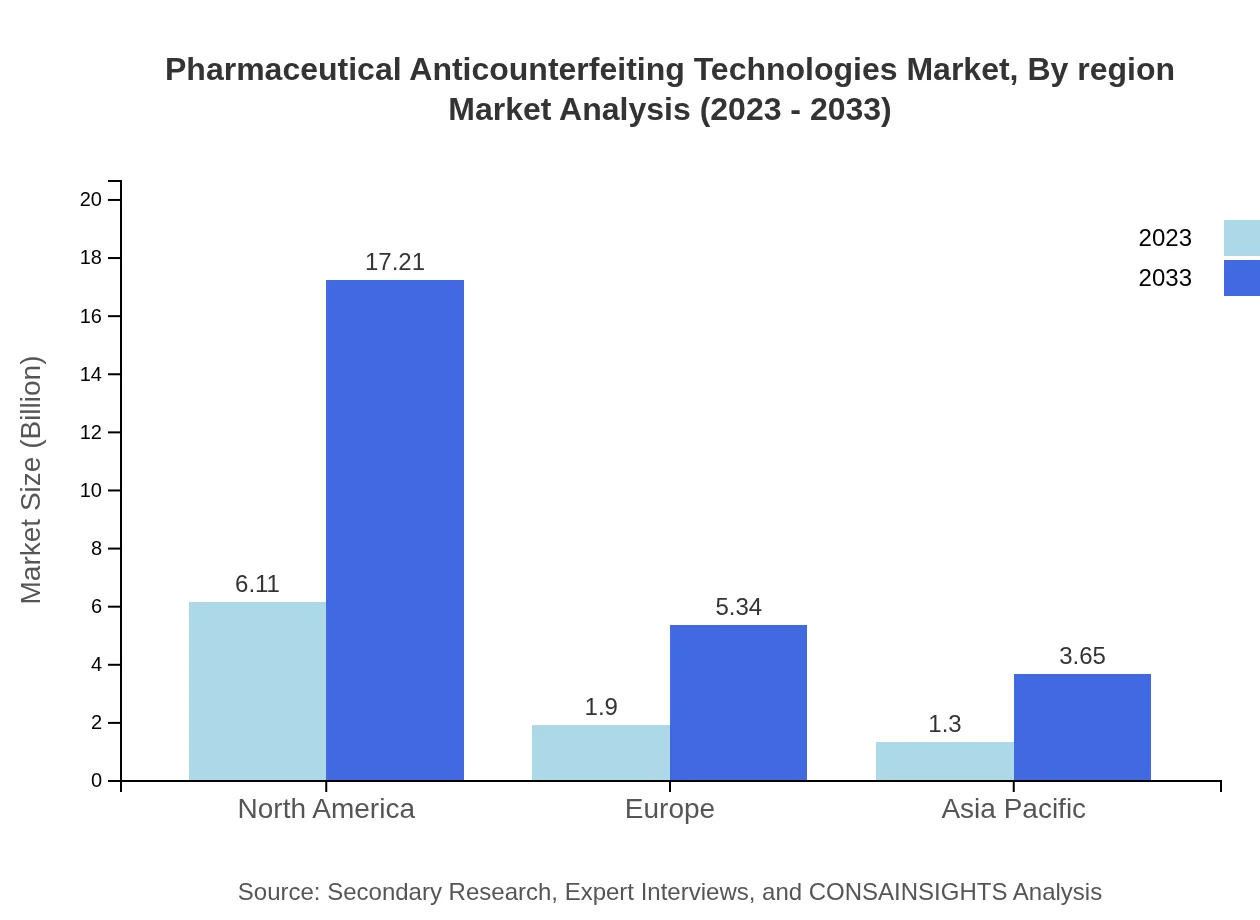
Europe is expected to see its market rise from $2.47 billion in 2023 to $6.97 billion by 2033, driven by stringent regulations on drug safety and a well-established healthcare system. Technological advancements in serialization and track-and-trace capabilities will further strengthen the market.Asia Pacific Pharmaceutical Anticounterfeiting Technologies Market Report:
The Asia Pacific region is projected to witness significant expansion in the Pharmaceutical Anticounterfeiting Technologies market, increasing from $1.85 billion in 2023 to $5.22 billion by 2033. This growth is driven by increasing investments in healthcare infrastructure and regulatory initiatives aimed at combating counterfeit drugs, particularly in emerging markets like India and China.North America Pharmaceutical Anticounterfeiting Technologies Market Report:
North America remains the largest market, with an estimated size of $3.03 billion in 2023, projected to grow to $8.55 billion by 2033. The robust regulatory environment and high investment in R&D by pharmaceutical players are key factors. The implementation of the Drug Supply Chain Security Act (DSCSA) continues to drive market growth in this region.South America Pharmaceutical Anticounterfeiting Technologies Market Report:
In South America, the market is expected to grow from $0.90 billion in 2023 to $2.54 billion by 2033. A growing healthcare sector and increased awareness of counterfeit threats are driving this growth. Governments in this region are becoming more proactive in implementing regulations to ensure the efficacy and safety of pharmaceuticals.Middle East & Africa Pharmaceutical Anticounterfeiting Technologies Market Report:
The market in the Middle East and Africa is anticipated to grow from $1.04 billion in 2023 to $2.92 billion by 2033. Growing healthcare budgets, coupled with rising awareness about the dangers of counterfeit medications, are facilitating market expansion in this region.Tell us your focus area and get a customized research report.
Pharmaceutical Anticounterfeiting Technologies Market Analysis By Technology
Authentication Technologies lead the segment with a market size of $6.11 billion in 2023, projected to reach $17.21 billion by 2033. Track and Trace Technologies and Serialization Technologies follow. Track and Trace Technologies will grow from $1.90 billion in 2023 to $5.34 billion by 2033. Serialization Technologies are also expected to reflect significant growth: from $1.30 billion to $3.65 billion in the same period.
Pharmaceutical Anticounterfeiting Technologies Market Analysis By Product Type
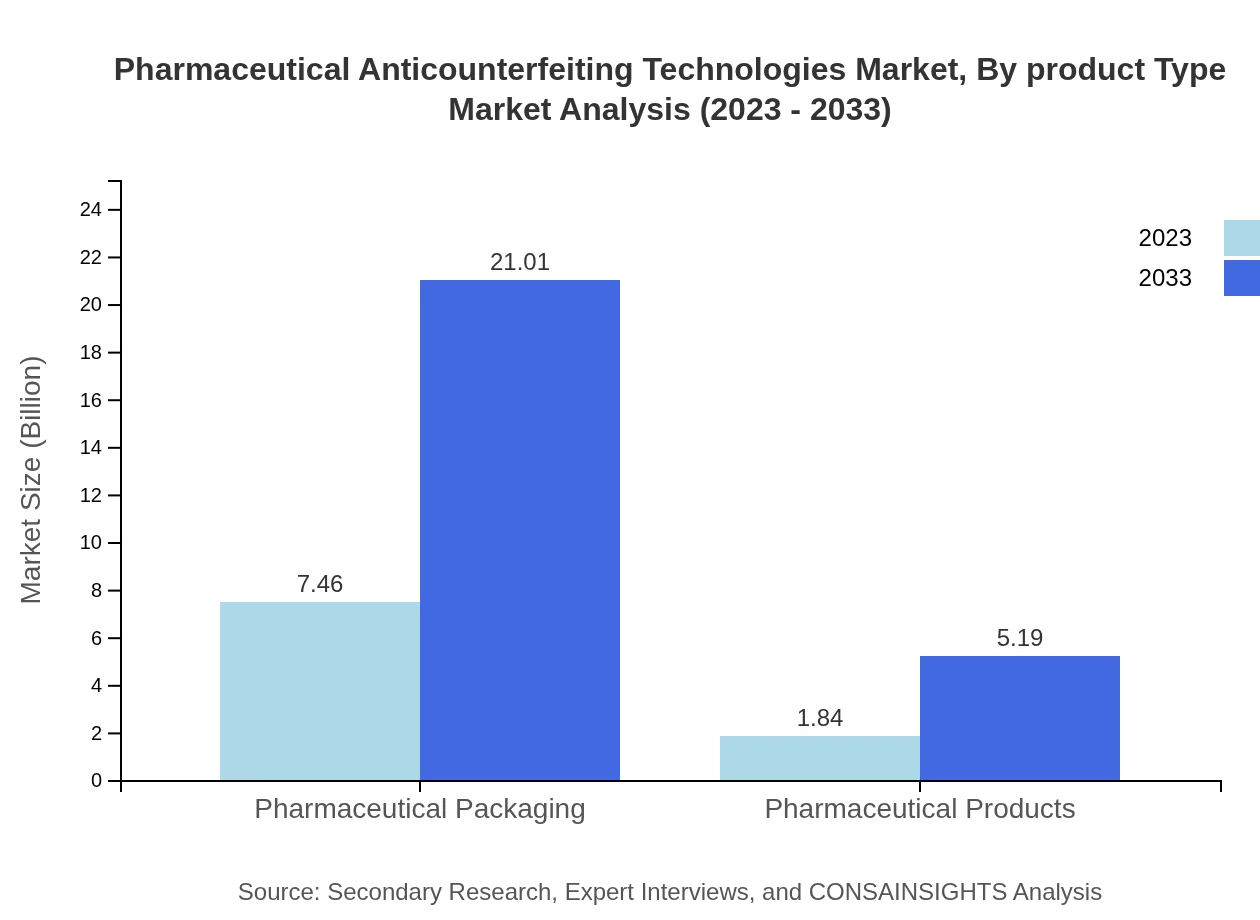
Pharmaceutical packaging dominates the market, expected to grow from $7.46 billion in 2023 to $21.01 billion by 2033. This is followed by pharmaceutical products, which are anticipated to increase from $1.84 billion to $5.19 billion. The increasing implementation of secure packaging solutions is enhancing market growth.
Pharmaceutical Anticounterfeiting Technologies Market Analysis By End User
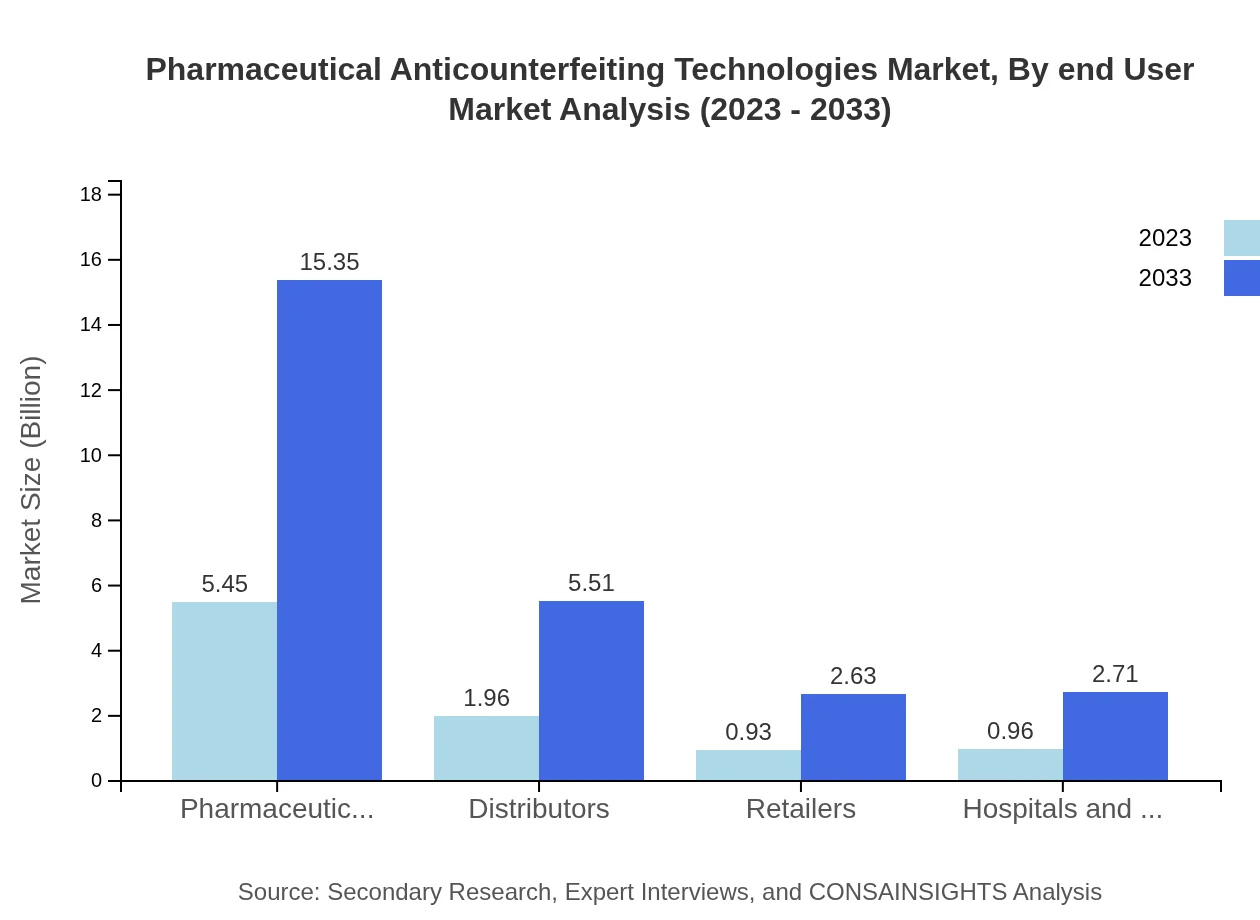
Pharmaceutical manufacturers hold a major share of the market, with a size of $5.45 billion in 2023 and a projected growth to $15.35 billion by 2033. Distributors and retailers follow, with distributors projected to increase from $1.96 billion to $5.51 billion. This growth in end-users highlights the broad adoption of anticounterfeiting technologies along the supply chain.
Pharmaceutical Anticounterfeiting Technologies Market Analysis By Region
Detailed regional analysis reflects key market opportunities and challenges across different regions. North America accounts for significant market share, driven by regulations and advanced technologies. Europe follows, emphasizing regulatory compliance. Growth prospects in Asia Pacific and South America reveal untapped opportunities, while the Middle East and Africa show potential for rapid growth due to increasing healthcare investments.
Pharmaceutical Anticounterfeiting Technologies Market Trends and Future Forecast
Tell us your focus area and get a customized research report.
Global Market Leaders and Top Companies in Pharmaceutical Anticounterfeiting Technologies Industry
Systech International:
A leader in the field of supply chain security, offering serialization and track-and-trace solutions to improve product integrity.Authenta:
Specializes in providing innovative authentication technologies that help pharmaceutical companies combat counterfeit products effectively.Zebra Technologies:
Offers comprehensive barcode and RFID solutions designed to enhance product traceability and protection against counterfeiting.PharmaSecure:
Develops mobile authentication solutions allowing for direct consumer engagement and product verification.We're grateful to work with incredible clients.









FAQs
What is the market size of pharmaceutical Anticounterfeiting Technologies?
The pharmaceutical anticounterfeiting technologies market is valued at approximately $9.3 billion in 2023, with a projected Compound Annual Growth Rate (CAGR) of 10.5% from 2023 to 2033.
What are the key market players or companies in this pharmaceutical Anticounterfeiting Technologies industry?
Key players in the pharmaceutical anticounterfeiting technologies industry include major pharmaceutical manufacturers, distributors, and innovators in authentication and serialization technologies, driving robust competition in this fast-evolving sector.
What are the primary factors driving the growth in the pharmaceutical Anticounterfeiting Technologies industry?
Growth in this industry is primarily driven by increasing incidences of drug counterfeiting, regulatory requirements for product tracking, and demand for enhanced consumer safety in pharmaceutical products.
Which region is the fastest Growing in the pharmaceutical Anticounterfeiting Technologies?
The fastest-growing region in the pharmaceutical anticounterfeiting technologies market is projected to be North America, expanding from $3.03 billion in 2023 to $8.55 billion by 2033, showcasing significant market potential.
Does ConsaInsights provide customized market report data for the pharmaceutical Anticounterfeiting Technologies industry?
Yes, ConsaInsights offers customized market report data tailored to specific needs in the pharmaceutical anticounterfeiting technologies industry, ensuring relevant and actionable insights for businesses.
What deliverables can I expect from this pharmaceutical Anticounterfeiting Technologies market research project?
Expect detailed market analysis reports, segmented data insights, regional trends, competitive landscape evaluations, and forecasts that guide strategic decisions in the pharmaceutical anticounterfeiting technologies sector.
What are the market trends of pharmaceutical Anticounterfeiting Technologies?
Current trends in the pharmaceutical anticounterfeiting technologies market include the rise of smart packaging solutions, increased investment in serialization technologies, and growing collaboration among stakeholders to enhance supply chain security.