Pharmaceutical Isolator Market Report
Published Date: 31 January 2026 | Report Code: pharmaceutical-isolator
Pharmaceutical Isolator Market Size, Share, Industry Trends and Forecast to 2033
This report provides a comprehensive analysis of the Pharmaceutical Isolator market from 2023 to 2033. It covers market size, trends, regional insights, segment analysis, and major players in the industry, offering key data for stakeholders and decision-makers.
| Metric | Value |
|---|---|
| Study Period | 2023 - 2033 |
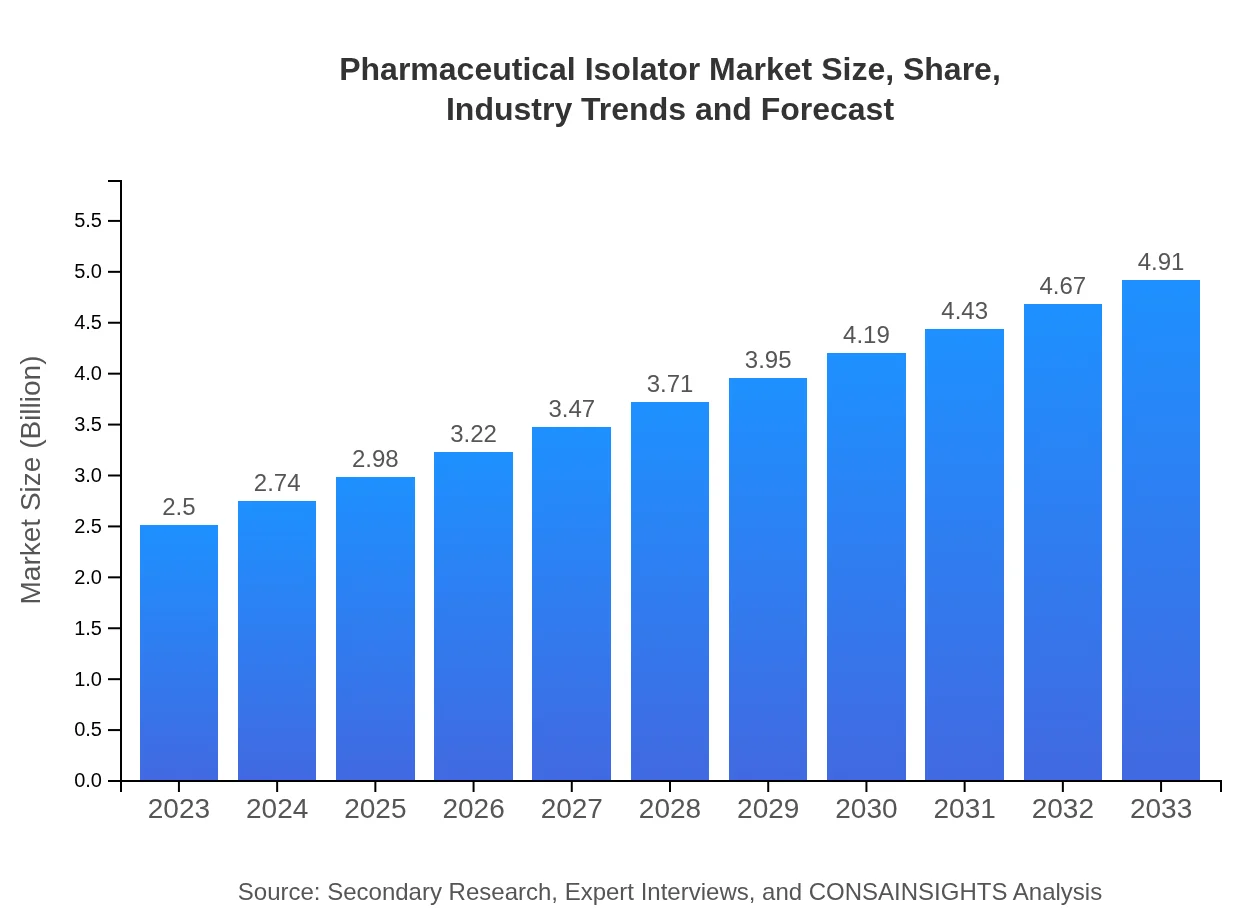
| 2023 Market Size | $2.50 Billion |
| CAGR (2023-2033) | 6.8% |
| 2033 Market Size | $4.91 Billion |
| Top Companies | Getinge AB, ATS Automation, SOPAT GmbH, NuAire Inc. |
| Last Modified Date | 31 January 2026 |
Pharmaceutical Isolator Market Overview
Customize Pharmaceutical Isolator Market Report market research report
- ✔ Get in-depth analysis of Pharmaceutical Isolator market size, growth, and forecasts.
- ✔ Understand Pharmaceutical Isolator's regional dynamics and industry-specific trends.
- ✔ Identify potential applications, end-user demand, and growth segments in Pharmaceutical Isolator
What is the Market Size & CAGR of Pharmaceutical Isolator market in 2033?
Pharmaceutical Isolator Industry Analysis
Pharmaceutical Isolator Market Segmentation and Scope
Tell us your focus area and get a customized research report.
Pharmaceutical Isolator Market Analysis Report by Region
Europe Pharmaceutical Isolator Market Report:
Europe's Pharmaceutical Isolator market is forecasted to increase notably from USD 0.77 billion in 2023 to USD 1.51 billion in 2033, fueled by stringent regulatory frameworks and a robust biopharmaceutical sector focused on innovation and quality assurance.Asia Pacific Pharmaceutical Isolator Market Report:
The Asia Pacific region's Pharmaceutical Isolator market is expected to show significant growth from USD 0.48 billion in 2023 to USD 0.93 billion in 2033, driven by increasing investments in pharmaceutical manufacturing and growing healthcare infrastructure in countries like China and India.North America Pharmaceutical Isolator Market Report:
North America represents a major portion of the global market, with expectations to rise from USD 0.86 billion in 2023 to USD 1.70 billion by 2033. This growth is primarily supported by the presence of leading pharmaceutical companies and rigorous regulatory standards.South America Pharmaceutical Isolator Market Report:
In South America, the market is projected to expand from USD 0.06 billion in 2023 to USD 0.13 billion by 2033. The growth is attributed to rising healthcare expenditure and the need for advanced manufacturing solutions in the pharmaceutical sector.Middle East & Africa Pharmaceutical Isolator Market Report:
The Middle East and Africa market is set to grow from USD 0.33 billion in 2023 to USD 0.64 billion in 2033, driven by an increasing need for quality assurance in medicine production and development of healthcare infrastructure.Tell us your focus area and get a customized research report.
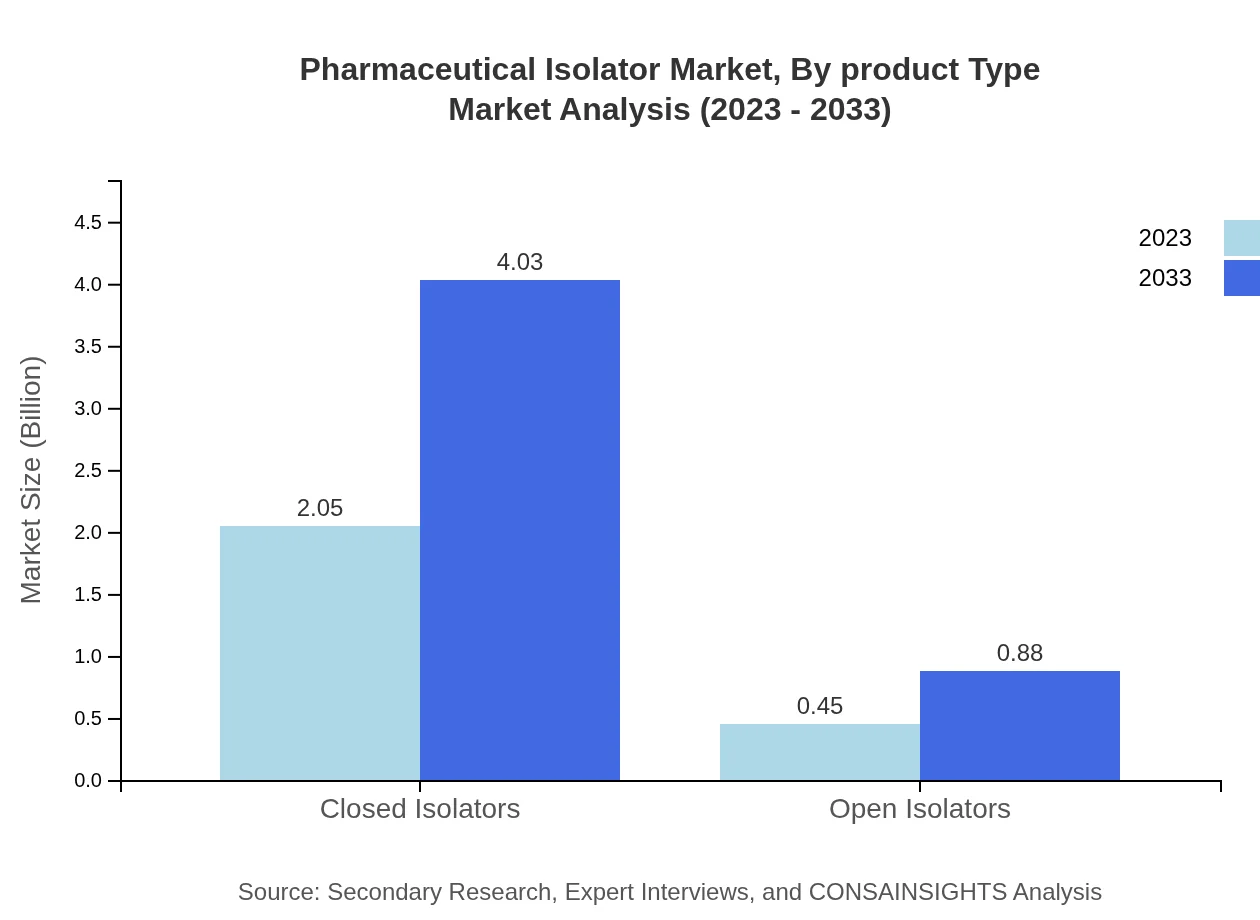
Pharmaceutical Isolator Market Analysis By Product Type
The Pharmaceutical Isolator market is predominantly driven by the closed isolators segment, expected to grow from USD 2.05 billion in 2023 to USD 4.03 billion in 2033, accounting for 82% of the market share by 2033. Open isolators, while gaining traction, are expected to grow at a slower rate, from USD 0.45 billion in 2023 to USD 0.88 billion in 2033, comprising 18% of the market.
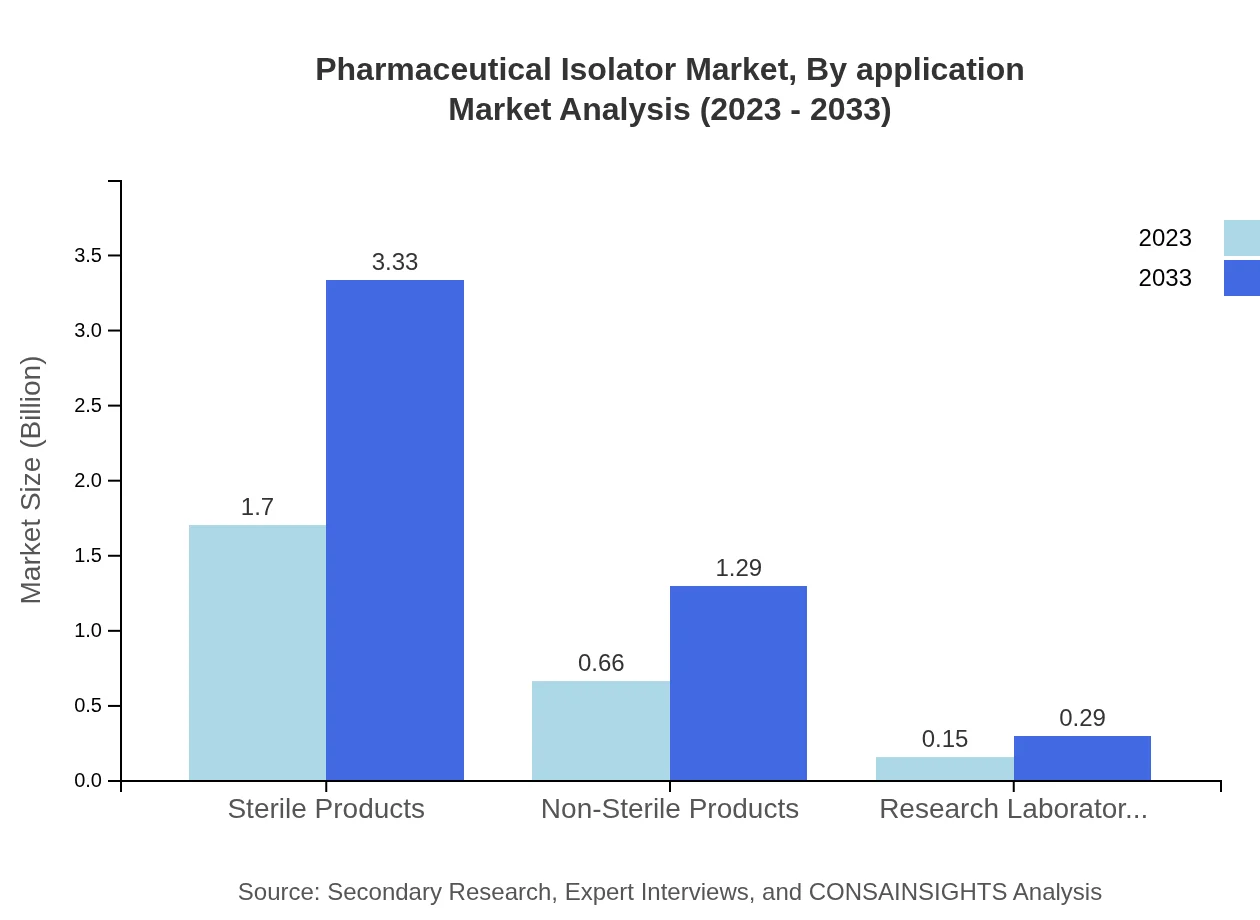
Pharmaceutical Isolator Market Analysis By Application
In terms of application, the market for sterile products significantly outpaces non-sterile options, with sterile products expected to grow from USD 1.70 billion in 2023 to USD 3.33 billion in 2033, maintaining a share of 67.82% throughout the forecasted period. Non-sterile products are also showing growth, anticipated to rise from USD 0.66 billion to USD 1.29 billion.
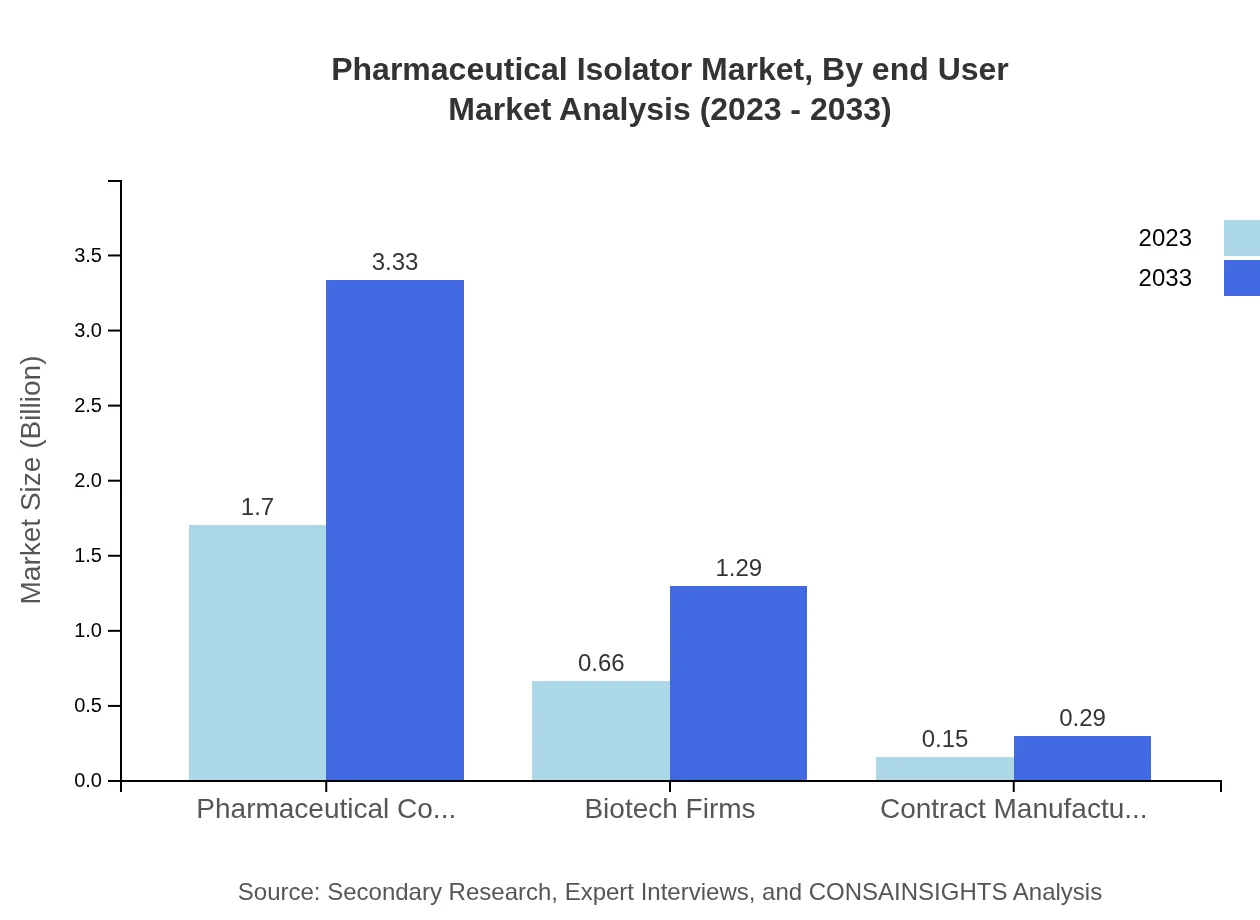
Pharmaceutical Isolator Market Analysis By End User
Pharmaceutical companies dominate the market, forecasted to grow from USD 1.70 billion to USD 3.33 billion, representing a steady market share of 67.82%. Biotech firms and contract manufacturing organizations are also important segments, expected to increase to USD 1.29 billion and USD 0.29 billion, respectively.
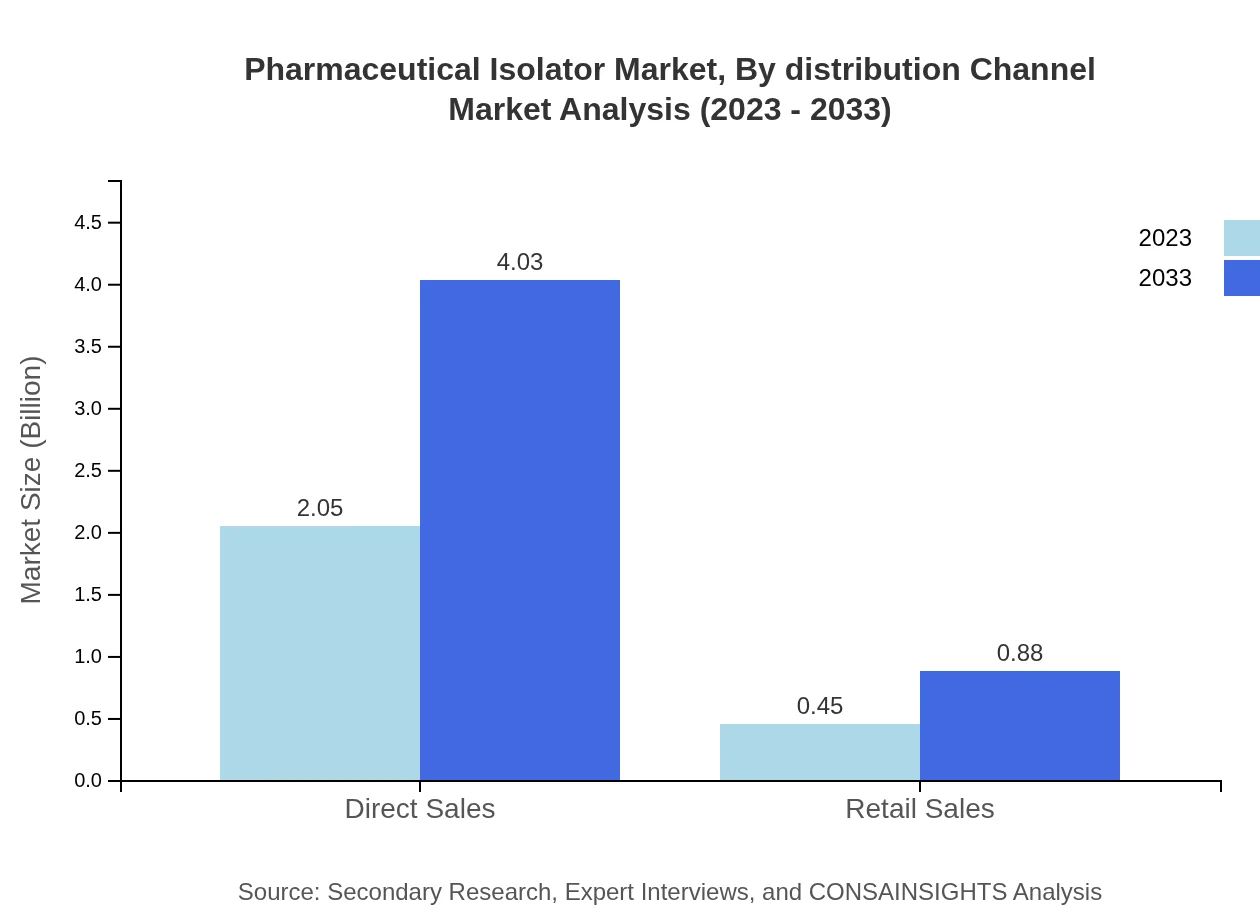
Pharmaceutical Isolator Market Analysis By Distribution Channel
The distribution channels for pharmaceutical isolators include direct sales and retail sales. Direct sales account for USD 2.05 billion in 2023 and are expected to rise to USD 4.03 billion by 2033, maintaining an 82.02% market share. Retail sales, while smaller, are projected to grow from USD 0.45 billion to USD 0.88 billion.
Pharmaceutical Isolator Market Trends and Future Forecast
Tell us your focus area and get a customized research report.
Global Market Leaders and Top Companies in Pharmaceutical Isolator Industry
Getinge AB:
Getinge AB is a leading global provider of innovative solutions for the medical technology, pharmaceutical, and biotechnology industries, offering high-quality isolators for sterile production.ATS Automation:
ATS Automation specializes in automation systems and is a prominent manufacturer of isolators for pharmaceutical applications, focusing on efficiency and streamlined production.SOPAT GmbH:
SOPAT GmbH develops and manufactures advanced isolator systems aimed at improving safety and sterility in pharmaceutical processes.NuAire Inc.:
NuAire Inc. provides laboratory equipment, including isolators designed for various sterile applications in the pharmaceutical field, emphasizing innovation and compliance.We're grateful to work with incredible clients.









FAQs
What is the market size of pharmaceutical Isolator?
The pharmaceutical isolator market is projected to grow from $2.5 billion in 2023 to significant levels by 2033, with a robust CAGR of 6.8%, highlighting a strong demand for isolator technology in the pharmaceutical sector.
What are the key market players or companies in this pharmaceutical Isolator industry?
Key players in the pharmaceutical isolator market include companies like Fedegari, Aseptic Technologies, and GEA Group, which dominate through innovative products and strong market presence.
What are the primary factors driving the growth in the pharmaceutical Isolator industry?
The growth of the pharmaceutical isolator market is driven by increasing regulations in cleanroom environments, rising demand for sterile products, and advancements in isolator technology, improving drug efficacy and patient safety.
Which region is the fastest Growing in the pharmaceutical Isolator?
North America is the fastest-growing region in the pharmaceutical isolator market, expected to grow from $0.86 billion in 2023 to $1.70 billion by 2033, reflecting rising healthcare investments and technological advancements.
Does ConsaInsights provide customized market report data for the pharmaceutical Isolator industry?
Yes, ConsaInsights offers customized market reports tailored to specific needs, allowing clients in the pharmaceutical isolator industry to access detailed market insights and trends that suit their strategic goals.
What deliverables can I expect from this pharmaceutical Isolator market research project?
Deliverables from the pharmaceutical isolator market research project include comprehensive market analysis reports, regional insights, competitive landscapes, and future growth forecasts segmented by type and application.
What are the market trends of pharmaceutical Isolator?
Current market trends in the pharmaceutical isolator industry include a shift towards automation, a focus on environmental sustainability, and increasing adoption of advanced technology to ensure product integrity and compliance.