Pharmacogenomics Market Report
Published Date: 31 January 2026 | Report Code: pharmacogenomics
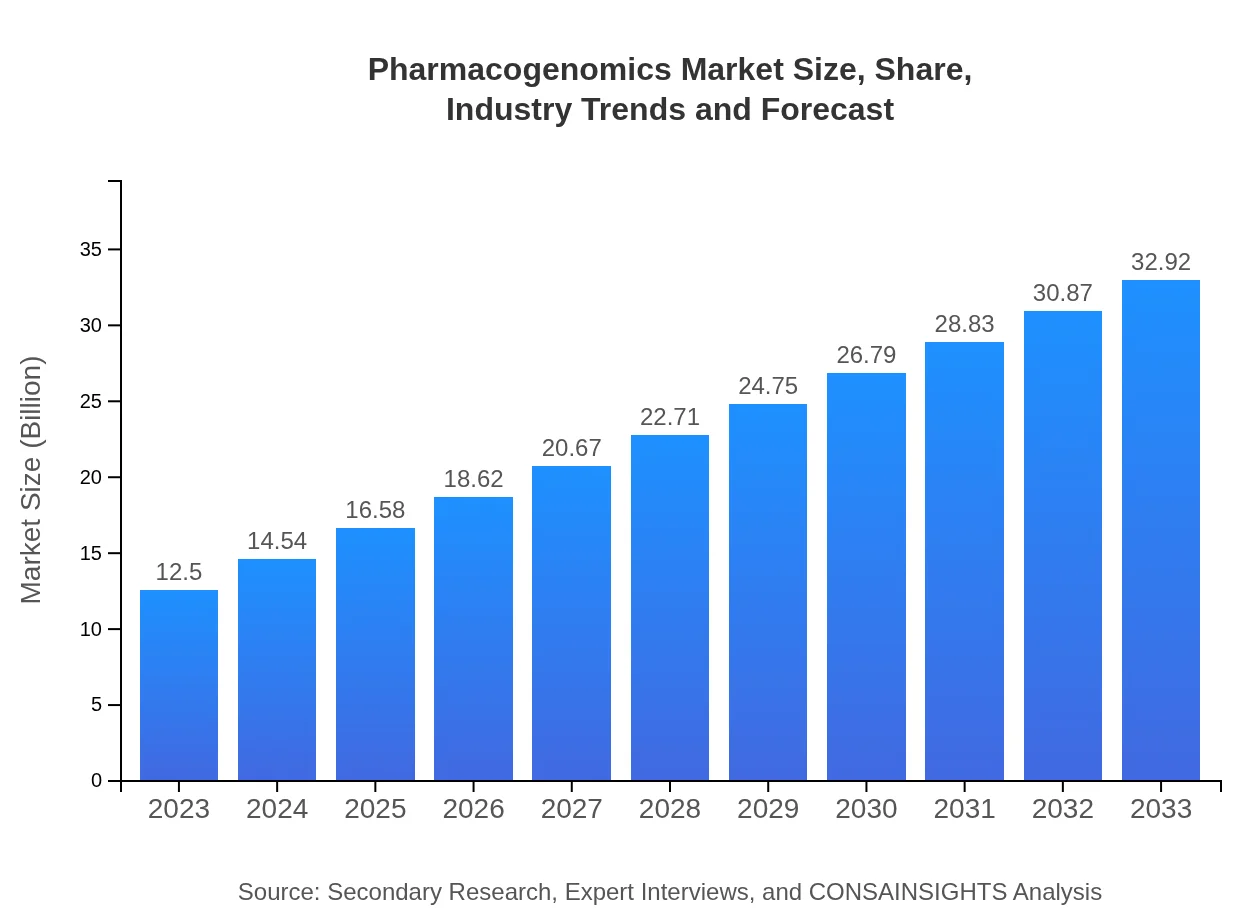
Pharmacogenomics Market Size, Share, Industry Trends and Forecast to 2033
This report provides a comprehensive overview of the pharmacogenomics market, including insights on market size, CAGR, regional analyses, technological advancements, and future forecasts from 2023 to 2033.
| Metric | Value |
|---|---|
| Study Period | 2023 - 2033 |
| 2023 Market Size | $12.50 Billion |
| CAGR (2023-2033) | 9.8% |
| 2033 Market Size | $32.92 Billion |
| Top Companies | Illumina, Inc., Thermo Fisher Scientific, Qiagen N.V., Roche Diagnostics |
| Last Modified Date | 31 January 2026 |
Pharmacogenomics Market Overview
Customize Pharmacogenomics Market Report market research report
- ✔ Get in-depth analysis of Pharmacogenomics market size, growth, and forecasts.
- ✔ Understand Pharmacogenomics's regional dynamics and industry-specific trends.
- ✔ Identify potential applications, end-user demand, and growth segments in Pharmacogenomics
What is the Market Size & CAGR of Pharmacogenomics market in 2023?
Pharmacogenomics Industry Analysis
Pharmacogenomics Market Segmentation and Scope
Tell us your focus area and get a customized research report.
Pharmacogenomics Market Analysis Report by Region
Europe Pharmacogenomics Market Report:
Europe's pharmacogenomics market is expected to grow from $3.72 billion in 2023 to $9.80 billion by 2033. The focus on research and development of genomic therapies and supportive regulatory frameworks enhance market prospects in this region.Asia Pacific Pharmacogenomics Market Report:
The Asia Pacific region's pharmacogenomics market is expected to grow from $2.63 billion in 2023 to $6.93 billion by 2033. This growth is fueled by increasing investments in healthcare infrastructure, a rise in chronic diseases, and a growing awareness of personalized medicine.North America Pharmacogenomics Market Report:
North America is the market leader, projected to expand from $4.25 billion in 2023 to $11.20 billion by 2033. The significant spending on healthcare and established infrastructure for biomedical research contribute to this robust growth.South America Pharmacogenomics Market Report:
In South America, the pharmacogenomics market is projected to grow from $0.53 billion in 2023 to $1.40 billion by 2033. The increasing adoption of technological advancements and the rising prevalence of genetic disorders drive this market.Middle East & Africa Pharmacogenomics Market Report:
The Middle East and Africa market is anticipated to grow from $1.36 billion in 2023 to $3.58 billion by 2033, with increasing awareness of genetic testing and healthcare investments shaping its trajectory.Tell us your focus area and get a customized research report.
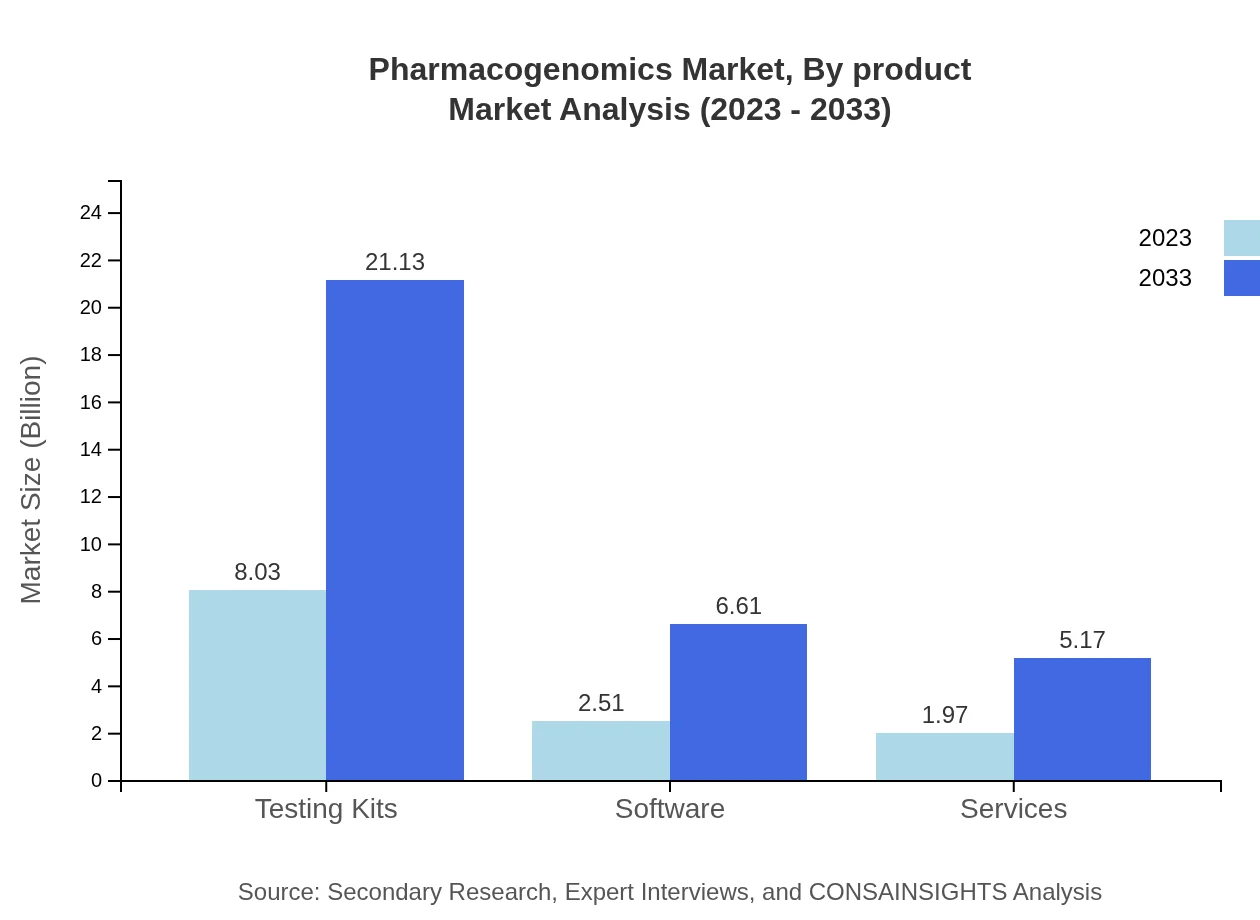
Pharmacogenomics Market Analysis By Product
The product segment of the pharmacogenomics market includes testing kits, software, and bioinformatics tools. Testing kits are the leading segment, projected to grow from $8.03 billion in 2023 to $21.13 billion by 2033. Software used for data analysis and interpretation will also see significant growth, from $2.51 billion to $6.61 billion in the same period.
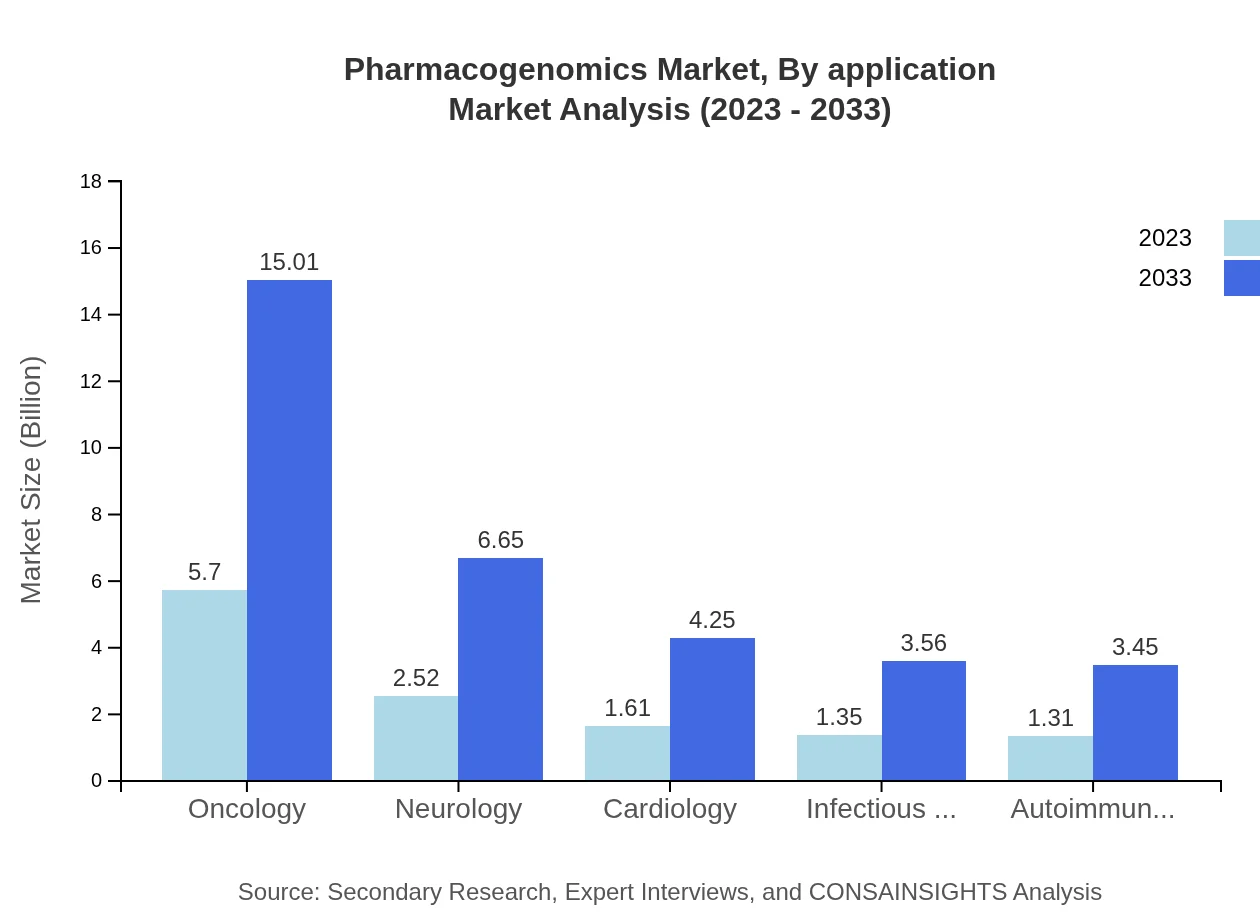
Pharmacogenomics Market Analysis By Application
Oncology is the largest application segment, expected to grow from $5.70 billion in 2023 to $15.01 billion by 2033, driven by increasing cancer prevalence and the demand for targeted therapies.
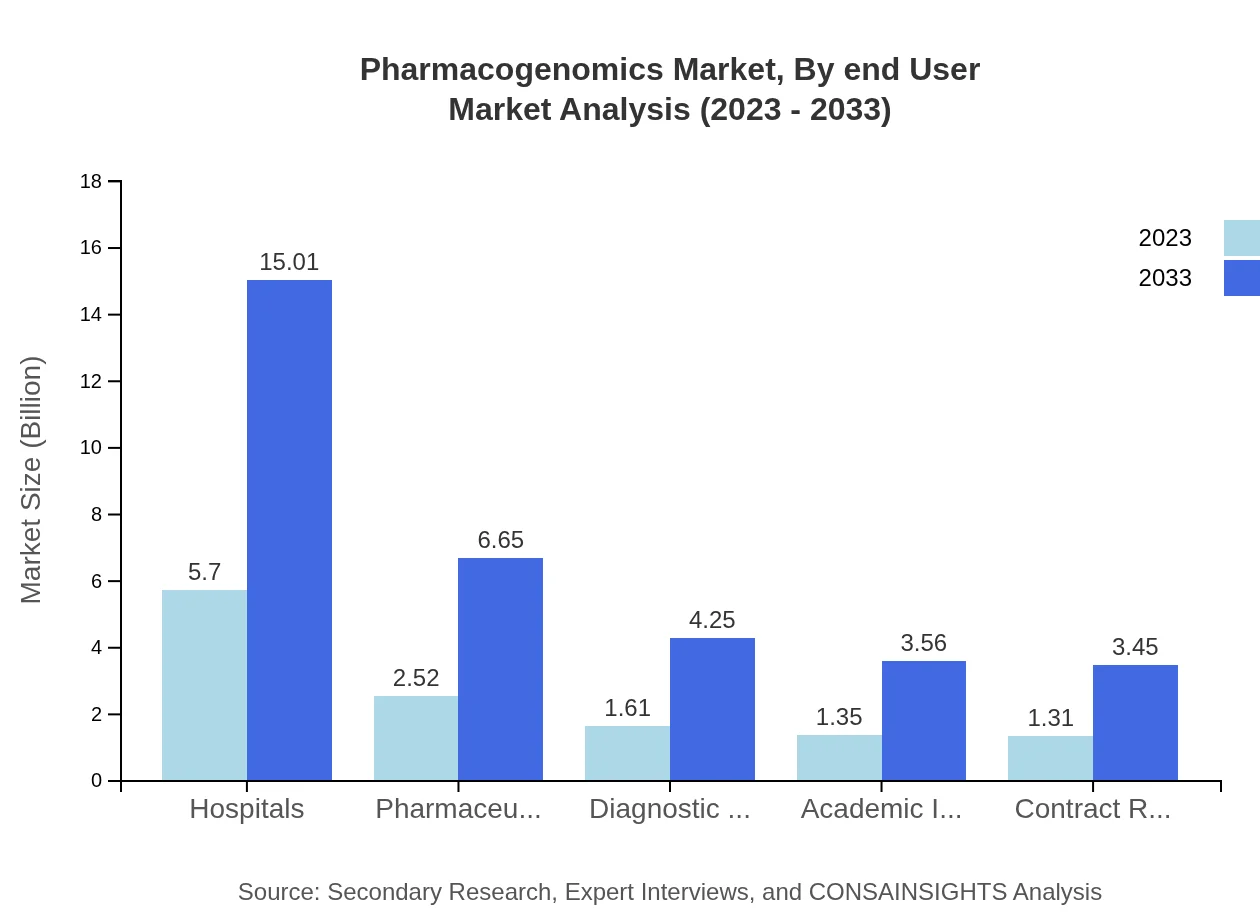
Pharmacogenomics Market Analysis By End User
Hospitals are the leading end-users, set to grow from $5.70 billion in 2023 to $15.01 billion by 2033, as more healthcare facilities adopt pharmacogenomic testing for personalized patient care.
Pharmacogenomics Market Analysis By Region
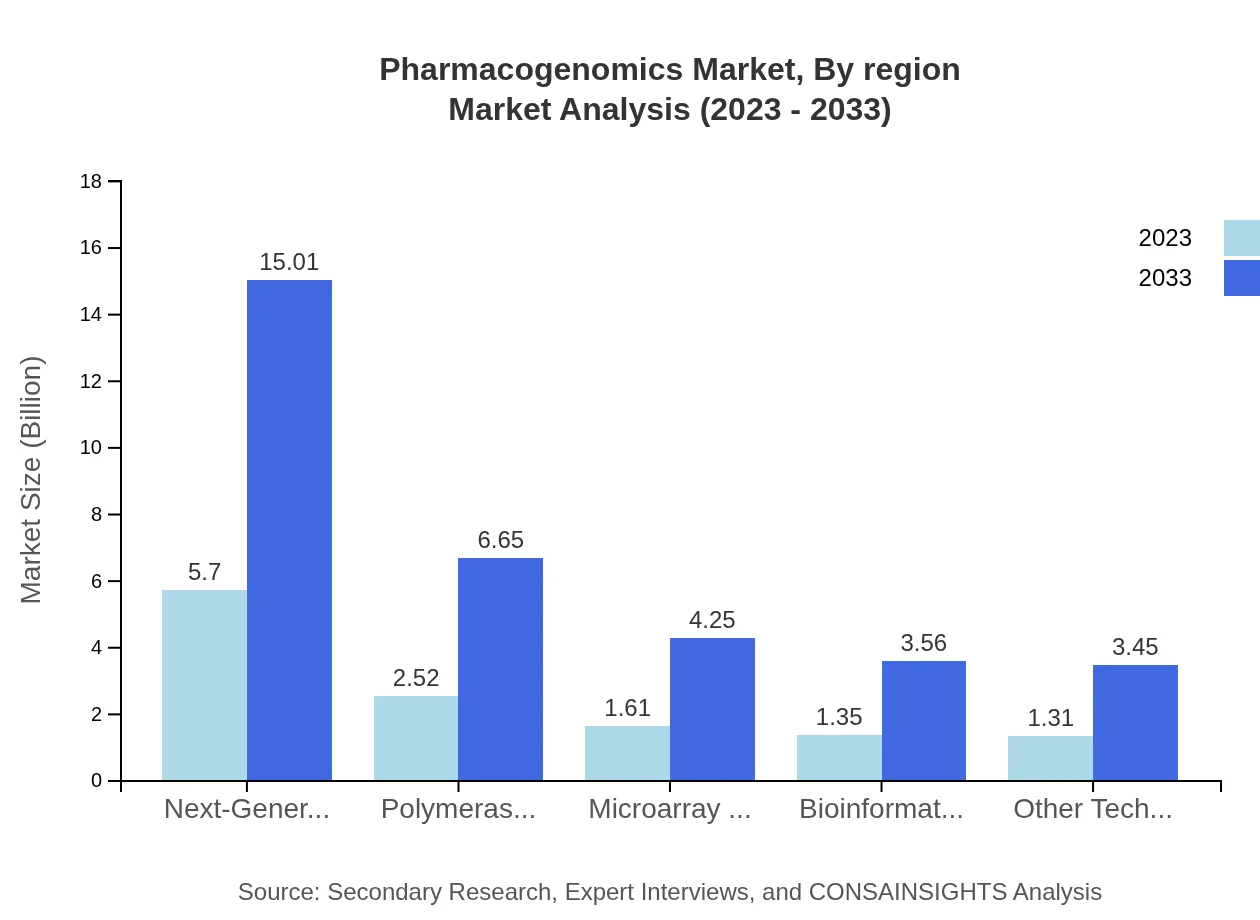
Technology types include Next-Generation Sequencing (NGS) and Polymerase Chain Reaction (PCR), among others. NGS is expected to dominate, growing from $5.70 billion in 2023 to $15.01 billion by 2033, indicative of its critical role in genomics research.
Pharmacogenomics Market Trends and Future Forecast
Tell us your focus area and get a customized research report.
Global Market Leaders and Top Companies in Pharmacogenomics Industry
Illumina, Inc.:
A global leader in genomics analysis, Illumina designs and manufactures devices for DNA sequencing and genotyping, driving advancements in precision medicine.Thermo Fisher Scientific:
A major player in the biotechnology space, Thermo Fisher offers a range of products and services in the areas of genetic sequencing and clinical diagnostics.Qiagen N.V.:
Qiagen is renowned for its sample and assay technologies, providing critical tools for genetic research and drug development in pharmacogenomics.Roche Diagnostics:
A leader in global healthcare, Roche Diagnostics excels in developing laboratory tests and diagnostic solutions, positioning it at the forefront of personalized medicine.We're grateful to work with incredible clients.









FAQs
What is the market size of pharmacogenomics?
The pharmacogenomics market is valued at approximately $12.5 billion in 2023, with a remarkable CAGR of 9.8%. By 2033, it is projected to expand significantly, reflecting continuous growth in personalized medicine and genomics.
What are the key market players or companies in the pharmacogenomics industry?
Key players in the pharmacogenomics industry include major pharmaceutical companies such as Roche, Illumina, and Siemens Healthineers. Additionally, biotech firms and specialized diagnostics companies are vital, ensuring innovation and development in genetic testing.
What are the primary factors driving the growth in the pharmacogenomics industry?
The growth of pharmacogenomics is primarily driven by advancements in genomics technology, increasing demand for personalized medicine, and rising cases of adverse drug reactions. Moreover, government support and funding for genomic research plays a crucial role.
Which region is the fastest Growing in the pharmacogenomics market?
North America is currently the fastest-growing region in the pharmacogenomics market, with its size projected to grow from $4.25 billion in 2023 to $11.20 billion by 2033. Europe and Asia Pacific also show significant growth potential.
Does ConsaInsights provide customized market report data for the pharmacogenomics industry?
Yes, ConsaInsights offers customized market report data tailored to specific needs in the pharmacogenomics industry. Clients can access insights relevant to market trends, competitive analysis, and tailored data reflecting their strategic objectives.
What deliverables can I expect from this pharmacogenomics market research project?
Expected deliverables from the pharmacogenomics market research project include comprehensive market analysis reports, trend forecasting, competitor benchmarking, and segmentation analysis across various parameters like region, technology, and application.
What are the market trends of pharmacogenomics?
Key trends in the pharmacogenomics market include the rise of Next-Generation Sequencing (growing from $5.70 billion in 2023), increasing utilization of testing kits, and the focus on integration of bioinformatics tools, enhancing personalized and precision medicine.