Pharmacovigilance And Drug Safety Software Market Report
Published Date: 31 January 2026 | Report Code: pharmacovigilance-and-drug-safety-software
Pharmacovigilance And Drug Safety Software Market Size, Share, Industry Trends and Forecast to 2033
This report provides an in-depth analysis of the Pharmacovigilance and Drug Safety Software market, covering insights on market size, growth forecasts, regional developments, and key industry players. The forecast period spans from 2023 to 2033, providing a comprehensive understanding of current trends and future projections.
| Metric | Value |
|---|---|
| Study Period | 2023 - 2033 |
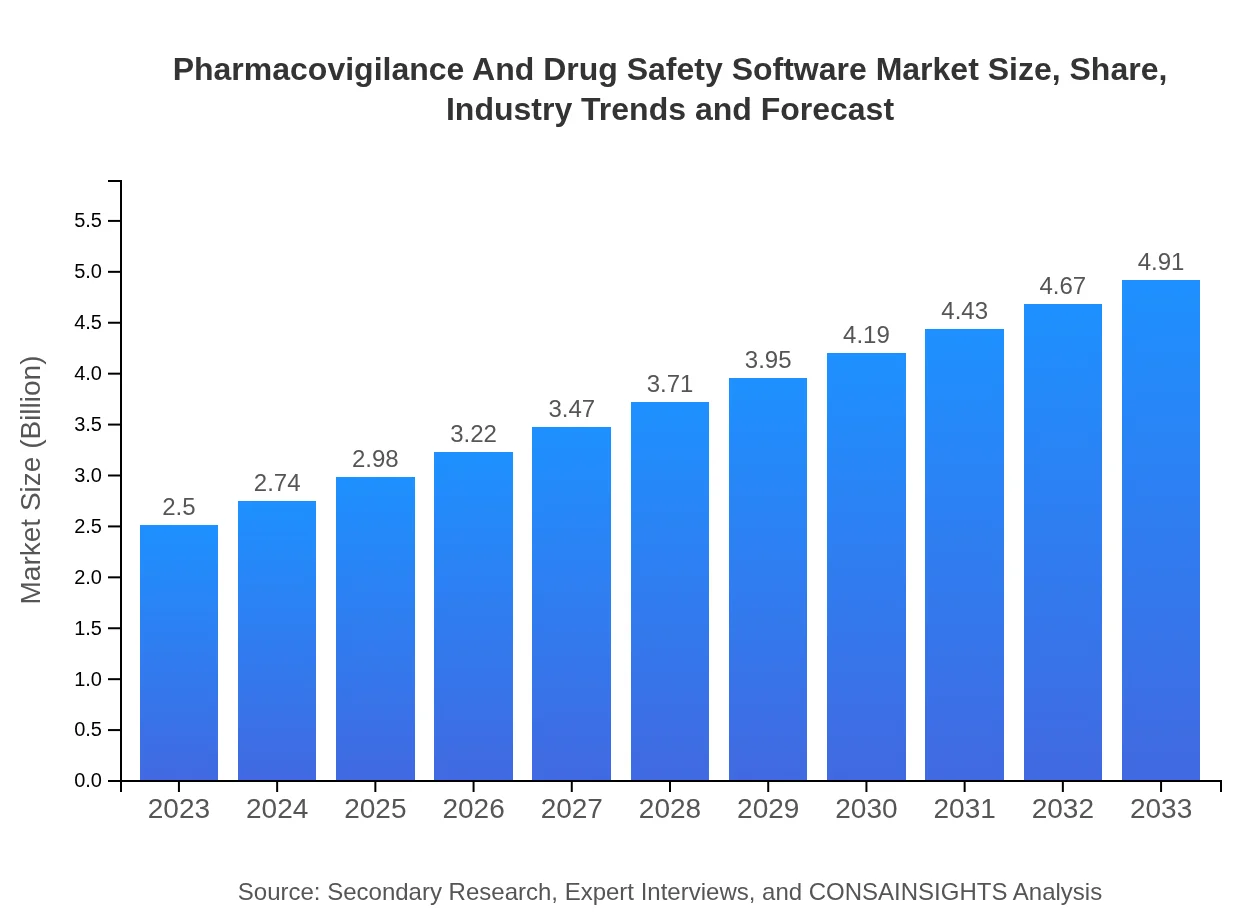
| 2023 Market Size | $2.50 Billion |
| CAGR (2023-2033) | 6.8% |
| 2033 Market Size | $4.91 Billion |
| Top Companies | Oracle, Veeva Systems, ArisGlobal, Dassault Systèmes |
| Last Modified Date | 31 January 2026 |
Pharmacovigilance And Drug Safety Software Market Overview
Customize Pharmacovigilance And Drug Safety Software Market Report market research report
- ✔ Get in-depth analysis of Pharmacovigilance And Drug Safety Software market size, growth, and forecasts.
- ✔ Understand Pharmacovigilance And Drug Safety Software's regional dynamics and industry-specific trends.
- ✔ Identify potential applications, end-user demand, and growth segments in Pharmacovigilance And Drug Safety Software
What is the Market Size & CAGR of Pharmacovigilance And Drug Safety Software market in 2023?
Pharmacovigilance And Drug Safety Software Industry Analysis
Pharmacovigilance And Drug Safety Software Market Segmentation and Scope
Tell us your focus area and get a customized research report.
Pharmacovigilance And Drug Safety Software Market Analysis Report by Region
Europe Pharmacovigilance And Drug Safety Software Market Report:
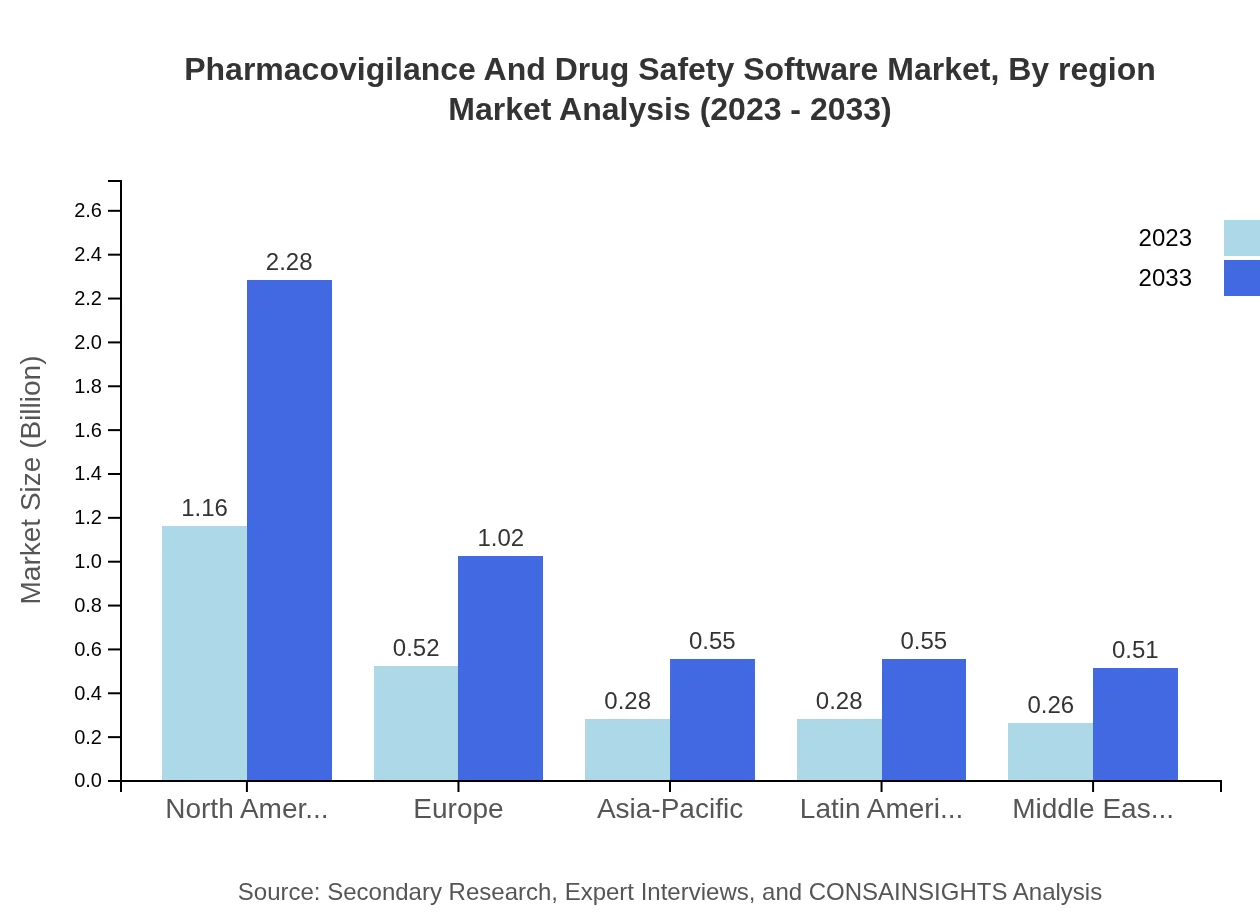
Europe is expected to witness substantial growth, moving from a market size of $0.77 billion in 2023 to $1.50 billion by 2033. The region's stringent pharmacovigilance regulations and the presence of key pharmaceutical companies bolster market potential.Asia Pacific Pharmacovigilance And Drug Safety Software Market Report:
The Asia-Pacific region has shown significant growth potential, with a market size of $0.43 billion anticipated to increase to $0.85 billion by 2033. The growing pharmaceutical sector and increasing regulatory demands are primary catalysts for this growth, alongside a rising focus on patient safety practices.North America Pharmacovigilance And Drug Safety Software Market Report:
North America leads the Pharmacovigilance and Drug Safety Software market, valued at $0.93 billion in 2023, projected to reach $1.83 billion in 2033. This growth is driven by regulatory demands, technological advancements, and a strong focus on ensuring drug safety.South America Pharmacovigilance And Drug Safety Software Market Report:
In South America, the market size is expected to grow from $0.13 billion in 2023 to $0.26 billion in 2033. Increasing investments in healthcare infrastructure and rising awareness of drug safety will significantly contribute to market expansion in this region.Middle East & Africa Pharmacovigilance And Drug Safety Software Market Report:
The Middle East and Africa region displays a growing interest in drug safety solutions, with a market size projected to grow from $0.24 billion in 2023 to $0.46 billion by 2033. Increasing collaboration among healthcare stakeholders to enhance drug safety practices will fuel market development.Tell us your focus area and get a customized research report.
Pharmacovigilance And Drug Safety Software Market Analysis By Software Type
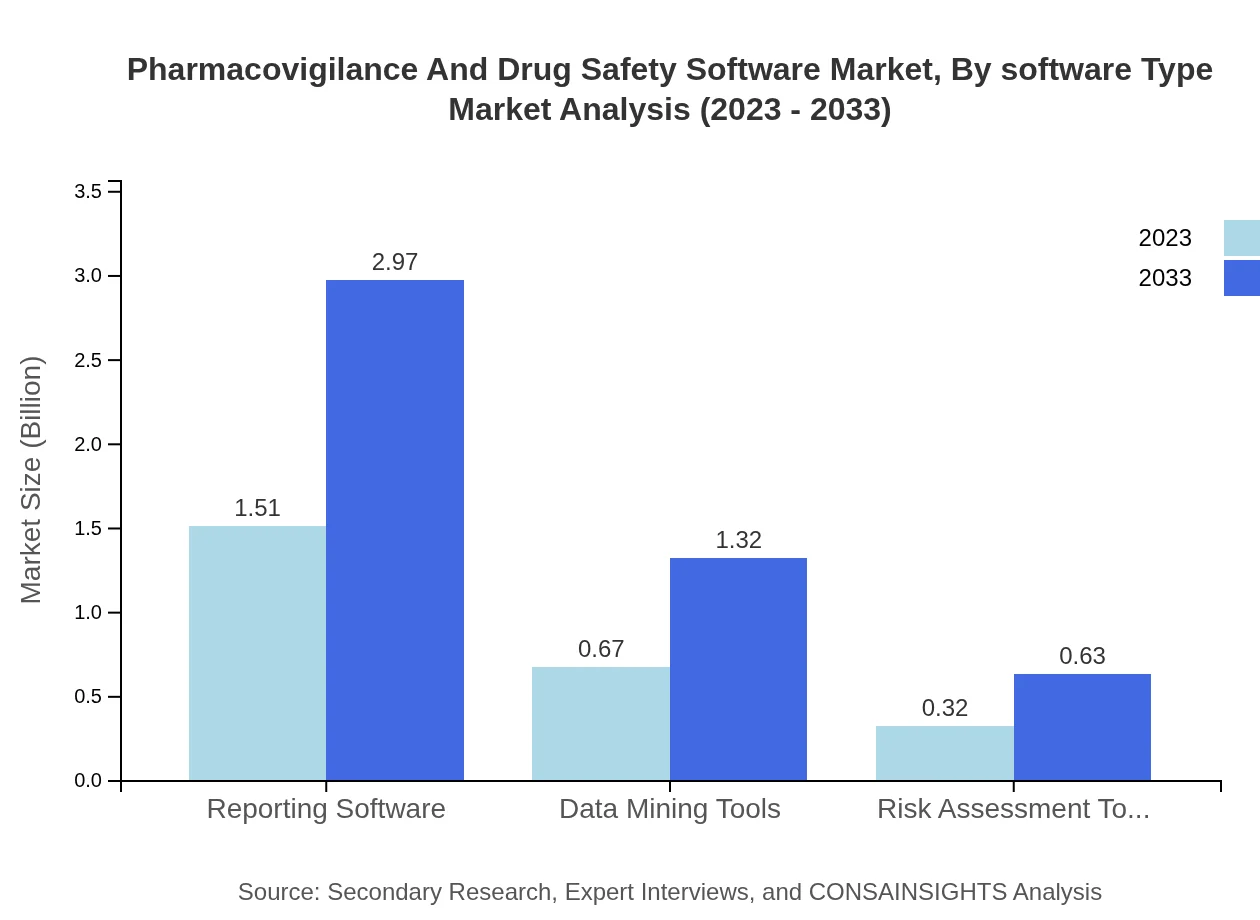
The software type segmentation of the market showcases robust demand for reporting software, which represents approximately 60.4% market share as of 2023. Data mining tools and risk assessment tools follow closely, with shares of 26.77% and 12.83%, respectively. The increasing focus on data analytics is driving the adoption of advanced data mining tools, facilitating better insights from adverse event reports.
Pharmacovigilance And Drug Safety Software Market Analysis By Deployment Mode
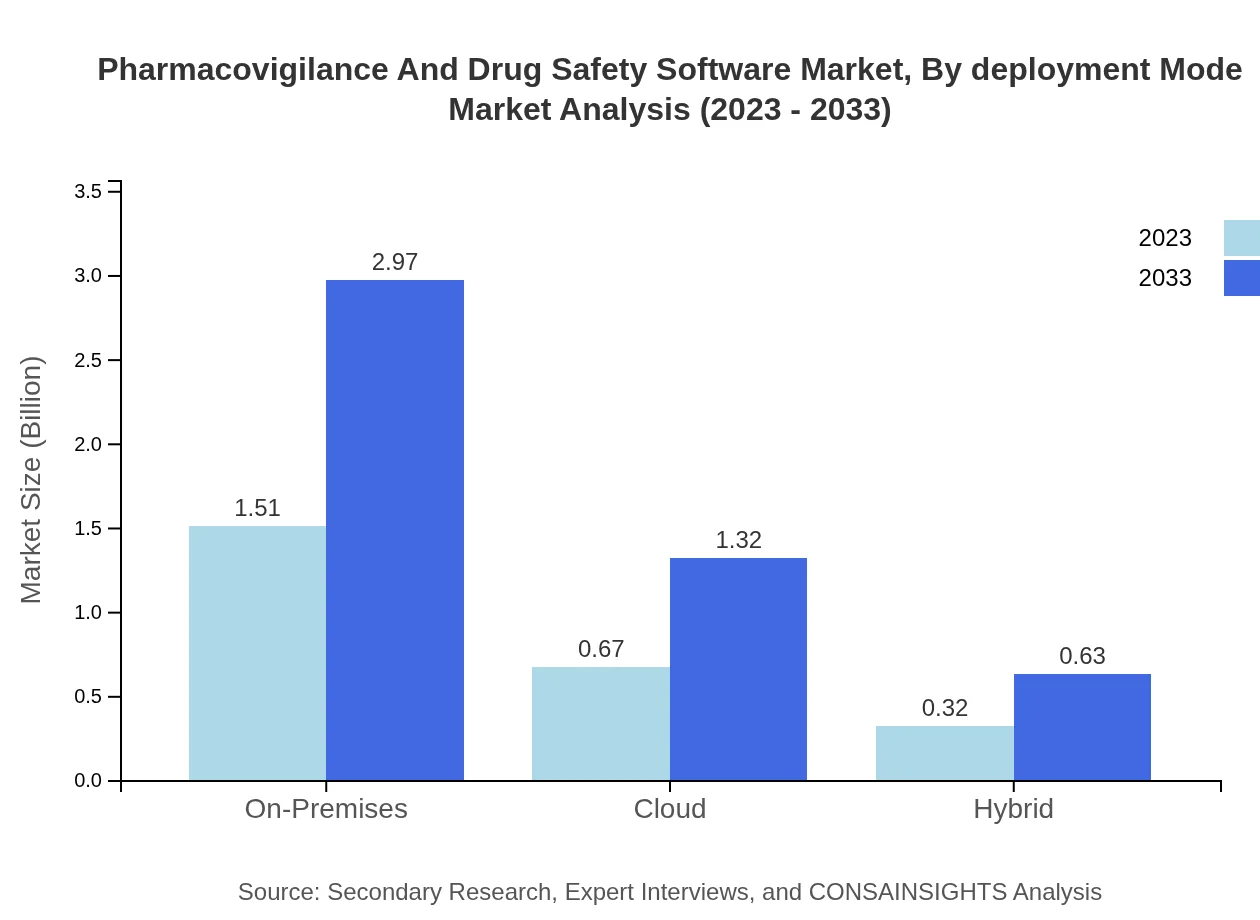
In terms of deployment modes, the on-premises solutions dominate the market with a share of 60.4%. However, there is a noticeable shift towards cloud-based solutions, projected to capture a significant share (26.77%) due to their cost-effectiveness and scalability. Hybrid models are also gaining traction, indicating a growing preference for flexible deployment options among enterprises.
Pharmacovigilance And Drug Safety Software Market Analysis By End User
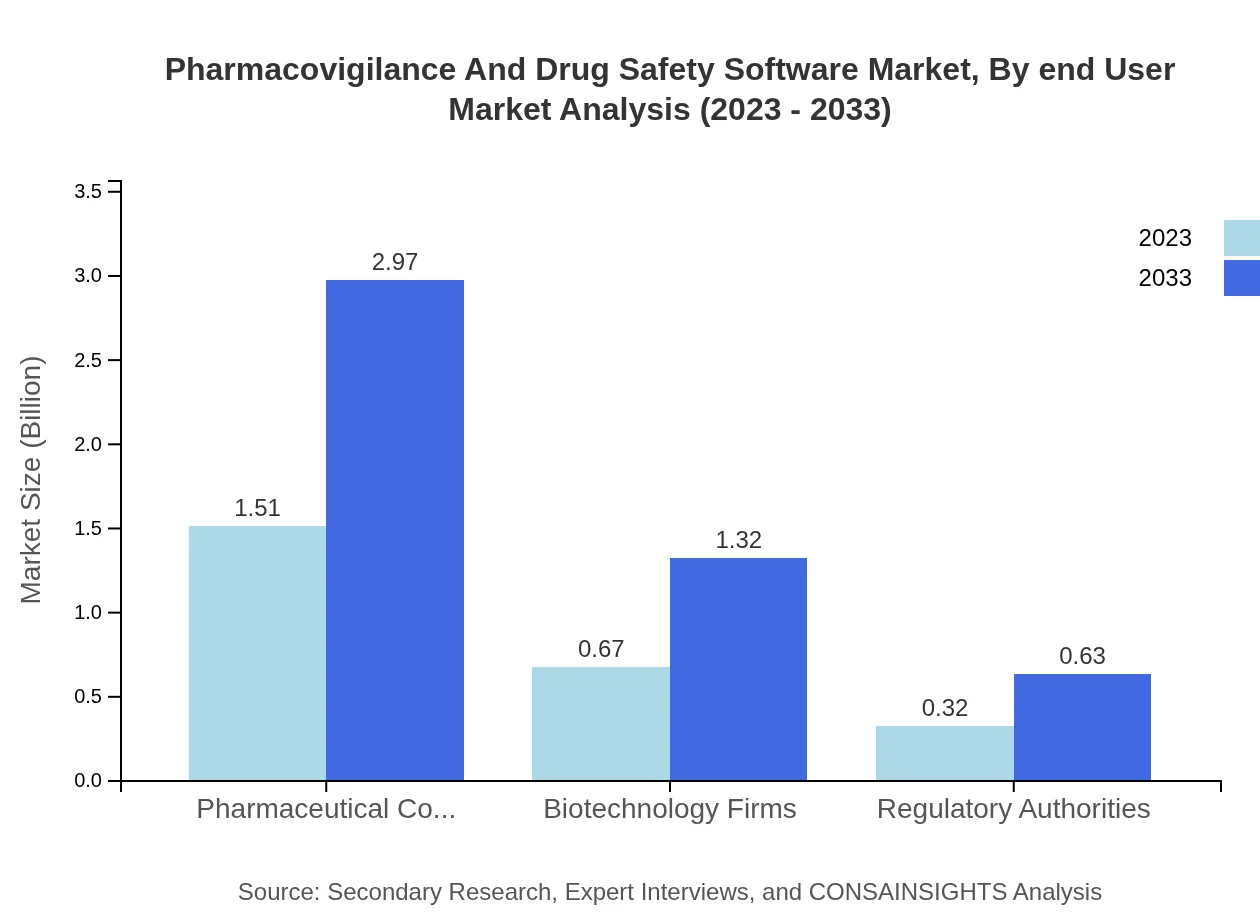
Pharmaceutical companies account for the largest segment in the end-user market with a 60.4% share in 2023. Biotechnology firms and regulatory authorities follow, with respective shares of 26.77% and 12.83%. As organizations emphasize compliance and risk management, the role of these software solutions in daily operations will continue to grow.
Pharmacovigilance And Drug Safety Software Market Analysis By Region
The regional analysis reveals North America as the market leader, being at $1.16 billion in 2023, while Europe and Asia-Pacific collectively present opportunities for growth rates surpassing historical levels. It provides essential insights into regional market dynamics and potential investment hotspots.
Pharmacovigilance And Drug Safety Software Market Trends and Future Forecast
Tell us your focus area and get a customized research report.
Global Market Leaders and Top Companies in Pharmacovigilance And Drug Safety Software Industry
Oracle:
Oracle is a leading provider of cloud-based solutions, including pharmacovigilance software, enabling efficient risk management and regulatory compliance for pharmaceutical companies worldwide.Veeva Systems:
Veeva Systems offers cloud-based software solutions tailored for the pharmaceutical industry, enhancing the efficiency of pharmacovigilance processes through integrated data management and reporting tools.ArisGlobal:
ArisGlobal provides advanced pharmacovigilance solutions designed for drug safety, regulatory compliance, and risk management, focusing on leveraging artificial intelligence for better analysis.Dassault Systèmes:
A leader in 3D design and engineering software, Dassault Systèmes also provides regulatory compliance software solutions to improve the drug safety monitoring process.We're grateful to work with incredible clients.









FAQs
What is the market size of pharmacovigilance And Drug Safety Software?
The pharmacovigilance and drug safety software market is projected to grow from $2.5 billion in 2023 to an estimated size significantly larger by 2033, with a Compound Annual Growth Rate (CAGR) of 6.8%.
What are the key market players or companies in this pharmacovigilance And Drug Safety Software industry?
Key players in the pharmacovigilance and drug safety software industry include major pharmaceutical companies and biotechnology firms, which represent approximately 60.4% and 26.77% market share, respectively, indicating their significant roles in the market.
What are the primary factors driving the growth in the pharmacovigilance And Drug Safety Software industry?
Key growth drivers for this industry include increasing regulatory requirements, growing awareness of drug safety among consumers, and advancements in technology that enhance data analytics and reporting capabilities in drug monitoring.
Which region is the fastest Growing in the pharmacovigilance And Drug Safety Software?
The fastest-growing region in the pharmacovigilance and drug safety software market is North America, where the market size is expected to increase from $0.93 billion in 2023 to $1.83 billion in 2033, reflecting a strong demand for advanced safety monitoring solutions.
Does ConsaInsights provide customized market report data for the pharmacovigilance And Drug Safety Software industry?
Yes, ConsaInsights provides customized market report data tailored to individual client needs in the pharmacovigilance and drug safety software industry, ensuring that stakeholders receive relevant insights for informed decision-making.
What deliverables can I expect from this pharmacovigilance And Drug Safety Software market research project?
Deliverables from this market research project typically include detailed reports, market forecasts, segment analysis, competitive landscape assessments, and insights into market trends for pharmacovigilance and drug safety software.
What are the market trends of pharmacovigilance And Drug Safety Software?
Current market trends for pharmacovigilance and drug safety software indicate a shift towards cloud-based solutions, increasing adoption of AI in drug monitoring, and heightened regulatory scrutiny, driving innovations and demand across the sector.