Pharmacovigilance Market Report
Published Date: 31 January 2026 | Report Code: pharmacovigilance
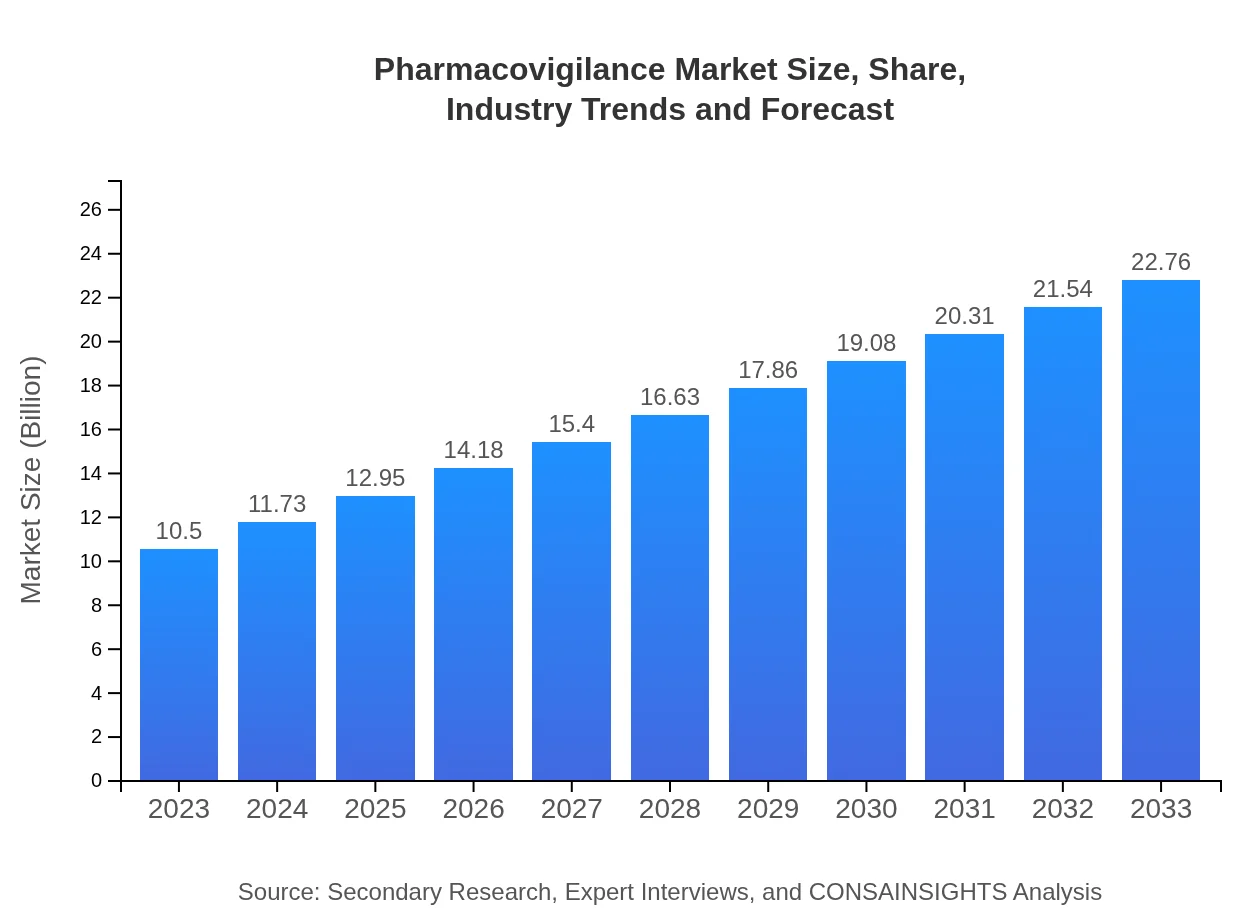
Pharmacovigilance Market Size, Share, Industry Trends and Forecast to 2033
This report examines the Pharmacovigilance market, providing insights and data from 2023 to 2033. It covers market size, growth forecasts, industry analysis, regional breakdowns, and the prominent technologies and players shaping the industry landscape.
| Metric | Value |
|---|---|
| Study Period | 2023 - 2033 |
| 2023 Market Size | $10.50 Billion |
| CAGR (2023-2033) | 7.8% |
| 2033 Market Size | $22.76 Billion |
| Top Companies | F. Hoffmann-La Roche Ltd., Johnson & Johnson Services, Inc., Wipro Limited, Accenture plc, Oracle Corporation |
| Last Modified Date | 31 January 2026 |
Pharmacovigilance Market Overview
Customize Pharmacovigilance Market Report market research report
- ✔ Get in-depth analysis of Pharmacovigilance market size, growth, and forecasts.
- ✔ Understand Pharmacovigilance's regional dynamics and industry-specific trends.
- ✔ Identify potential applications, end-user demand, and growth segments in Pharmacovigilance
What is the Market Size & CAGR of the Pharmacovigilance market in 2023?
Pharmacovigilance Industry Analysis
Pharmacovigilance Market Segmentation and Scope
Tell us your focus area and get a customized research report.
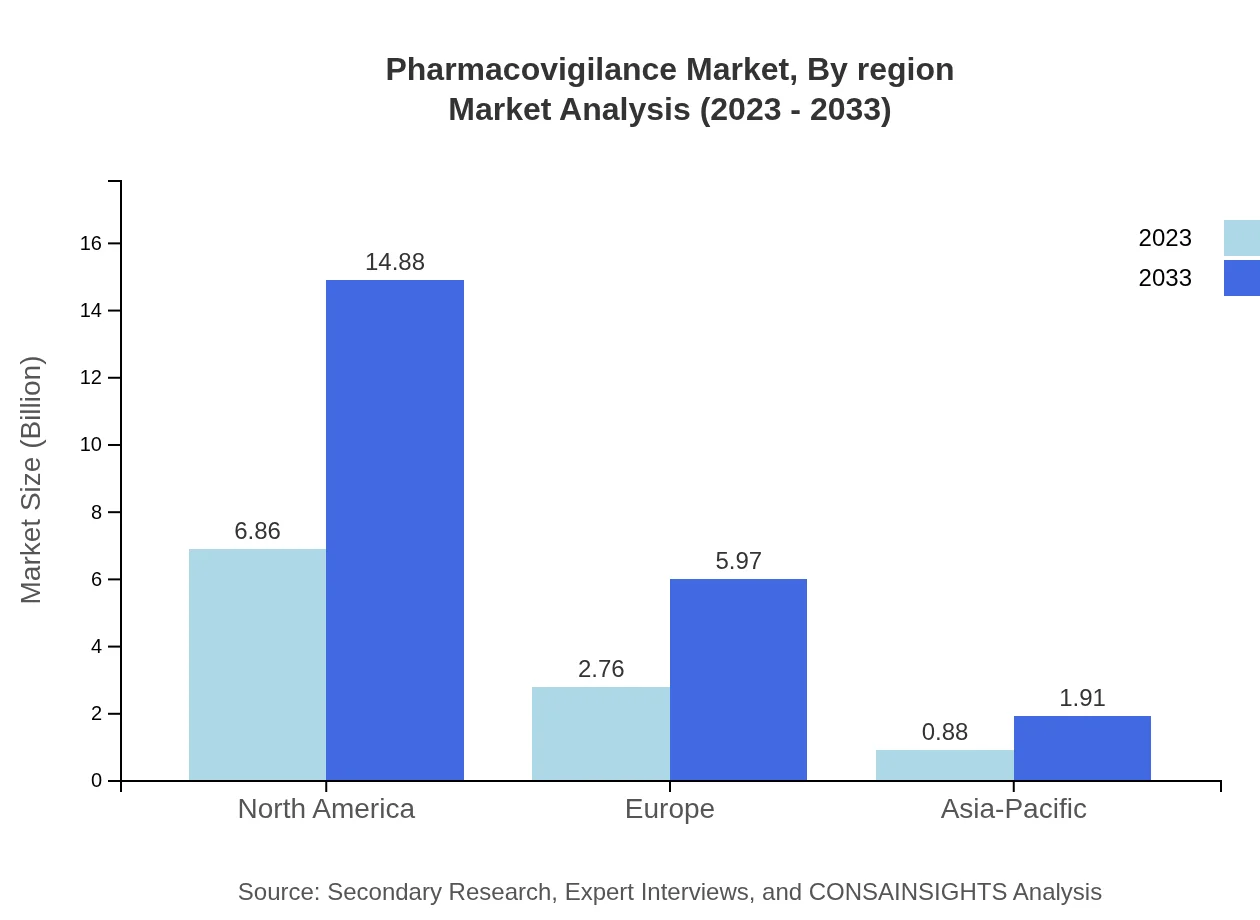
Pharmacovigilance Market Analysis Report by Region
Europe Pharmacovigilance Market Report:
In Europe, the market size is anticipated to expand from $3.32 billion in 2023 to $7.19 billion in 2033. This region is characterized by strong regulatory frameworks and an emphasis on safety protocols. The increasing number of adverse event reports is driving growth within European pharmacovigilance systems.Asia Pacific Pharmacovigilance Market Report:
The Pharmacovigilance market in Asia-Pacific is expected to grow from $1.82 billion in 2023 to $3.94 billion in 2033. This growth is supported by increasing regulatory initiatives, a rise in clinical trials, and enhanced safety protocols across pharmaceutical firms. Countries like India and China are emerging as significant players in this domain due to their expanding pharmaceutical sectors.North America Pharmacovigilance Market Report:
The North American market remains the largest player in the Pharmacovigilance sector, valued at $3.86 billion in 2023 with expectations to reach $8.37 billion by 2033. The U.S. is a major contributor due to stringent regulations, a robust pharmaceutical industry, and advancements in technology. The high level of awareness regarding drug safety significantly propels market growth in this region.South America Pharmacovigilance Market Report:
In South America, the Pharmacovigilance market is projected to grow from $0.76 billion in 2023 to $1.64 billion in 2033. The demand is being driven by increasing healthcare investments and the need for better regulatory oversight on drug safety. Brazil and Argentina are leading the adoption of pharmacovigilance practices.Middle East & Africa Pharmacovigilance Market Report:
The Middle East and Africa market is expected to grow from $0.75 billion in 2023 to $1.62 billion in 2033. Growth factors include increasing healthcare expenditure and the need for established safety monitoring systems. Emerging markets like South Africa and the UAE are particularly influential in this growth trajectory.Tell us your focus area and get a customized research report.
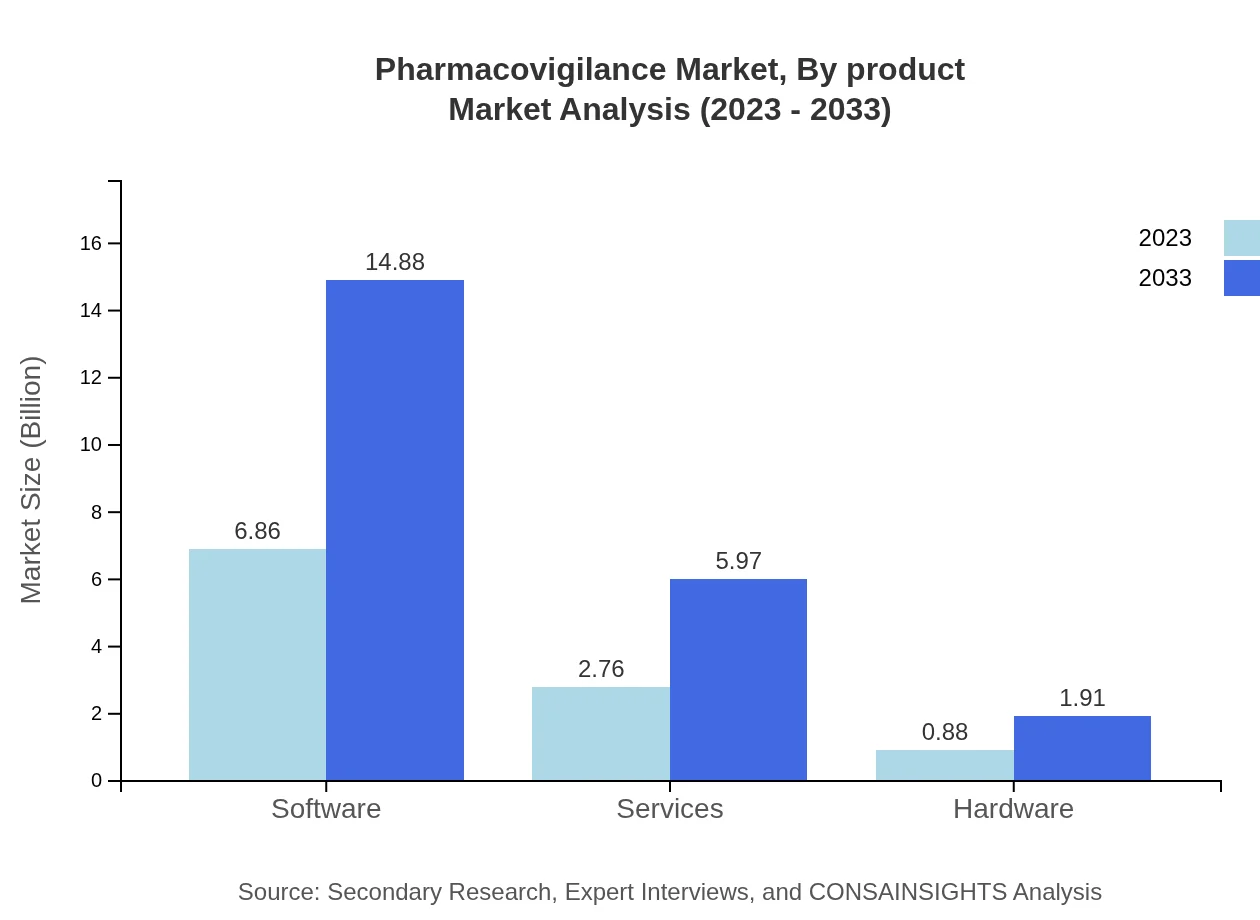
Pharmacovigilance Market Analysis By Product
The Pharmacovigilance market by product is primarily segmented into software, services, and hardware. Software is the leading segment, expected to grow significantly due to increased automation in monitoring systems. Services, including consulting and integrated solutions, form the second significant category, as organizations seek expert knowledge to navigate complex regulations. Hardware solutions support the technological backbone of monitoring systems but represent a smaller portion of the market.
Pharmacovigilance Market Analysis By Application
The market is segmented into applications such as drug safety and risk management. Drug safety continues to drive market growth as organizations prioritize ensuring patient safety. Risk management applications, which focus on the proactive identification of potential drug risks, are also gaining traction amid increasing regulatory scrutiny.
Pharmacovigilance Market Analysis By End User
End-users are primarily categorized into pharmaceutical companies, biotechnology firms, and regulatory agencies. Pharmaceutical companies dominate the market due to their vast portfolios of drugs requiring extensive safety monitoring. Biotechnology firms also contribute significantly, particularly in developing new therapies.
Pharmacovigilance Market Analysis By Region
The market analysis by region highlights North America as the dominant player, while Europe follows closely. Asia-Pacific is emerging with substantial growth potential driven by investments in healthcare. Latin America and the Middle East and Africa present smaller markets but are expanding as regional regulations improve.
Pharmacovigilance Market Analysis By Technology
Technological advancements play a critical role in pharmacovigilance. The adoption of artificial intelligence, big data analytics, and cloud computing is redefining traditional practices. AI is particularly valuable for processing large datasets, identifying trends, and automating reporting, which enhances efficiency and compliance.
Pharmacovigilance Market Trends and Future Forecast
Tell us your focus area and get a customized research report.
Global Market Leaders and Top Companies in Pharmacovigilance Industry
F. Hoffmann-La Roche Ltd.:
A global leader in pharmaceuticals, Roche is dedicated to enhancing drug safety through innovative pharmacovigilance practices.Johnson & Johnson Services, Inc.:
A diversified global healthcare leader, J&J implements robust pharmacovigilance systems across its vast range of products.Wipro Limited:
An IT services firm providing technological solutions to support pharmacovigilance and enhancing drug safety monitoring.Accenture plc:
Through consulting and technology services, Accenture aids biopharma companies in optimizing their pharmacovigilance operations.Oracle Corporation:
Offers cloud-based software solutions that advance compliance and automated reporting in pharmacovigilance.We're grateful to work with incredible clients.









FAQs
What is the market size of pharmacovigilance?
The pharmacovigilance market is valued at approximately $10.5 billion in 2023, with a projected CAGR of 7.8% from 2023 to 2033. This robust growth indicates a strong demand for safety monitoring in the pharmaceutical industry.
What are the key market players or companies in this pharmacovigilance industry?
Key players in the pharmacovigilance industry include major pharmaceutical companies, biotechnology firms, and regulatory agencies. Companies such as Pfizer, Merck, and Biogen lead in this sector by developing innovative drug safety solutions and compliance strategies.
What are the primary factors driving the growth in the pharmacovigilance industry?
Key growth drivers for the pharmacovigilance industry include increasing drug approvals, stringent regulatory frameworks, and a growing focus on patient safety. Furthermore, advancements in technology like AI and big data analytics are revolutionizing how drugs are monitored post-marketing.
Which region is the fastest Growing in the pharmacovigilance?
North America is the fastest-growing region in the pharmacovigilance market, projected to expand from $3.86 billion in 2023 to $8.37 billion by 2033. The region's regulatory environment and advanced healthcare systems contribute significantly to this growth.
Does ConsaInsights provide customized market report data for the pharmacovigilance industry?
Yes, ConsaInsights offers customized market report data tailored for the pharmacovigilance industry. Clients can request specific insights and analysis based on their unique needs, ensuring the information is relevant and actionable.
What deliverables can I expect from this pharmacovigilance market research project?
Deliverables from a pharmacovigilance market research project typically include comprehensive market analysis, competitive landscape assessments, growth forecasts, and regional performance data. Clients will gain insights essential for strategic planning.
What are the market trends of pharmacovigilance?
Key trends in the pharmacovigilance market include the increasing integration of AI and machine learning, a shift towards real-time data monitoring, and enhanced regulatory compliance measures. This evolution is geared towards improving drug safety and efficacy globally.