Photopheresis Products Market Report
Published Date: 31 January 2026 | Report Code: photopheresis-products
Photopheresis Products Market Size, Share, Industry Trends and Forecast to 2033
This report analyzes the Photopheresis Products market from 2023 to 2033, covering market trends, size, and insights across various segments and regions. It provides a forecast of growth opportunities and the competitive landscape influencing the industry.
| Metric | Value |
|---|---|
| Study Period | 2023 - 2033 |
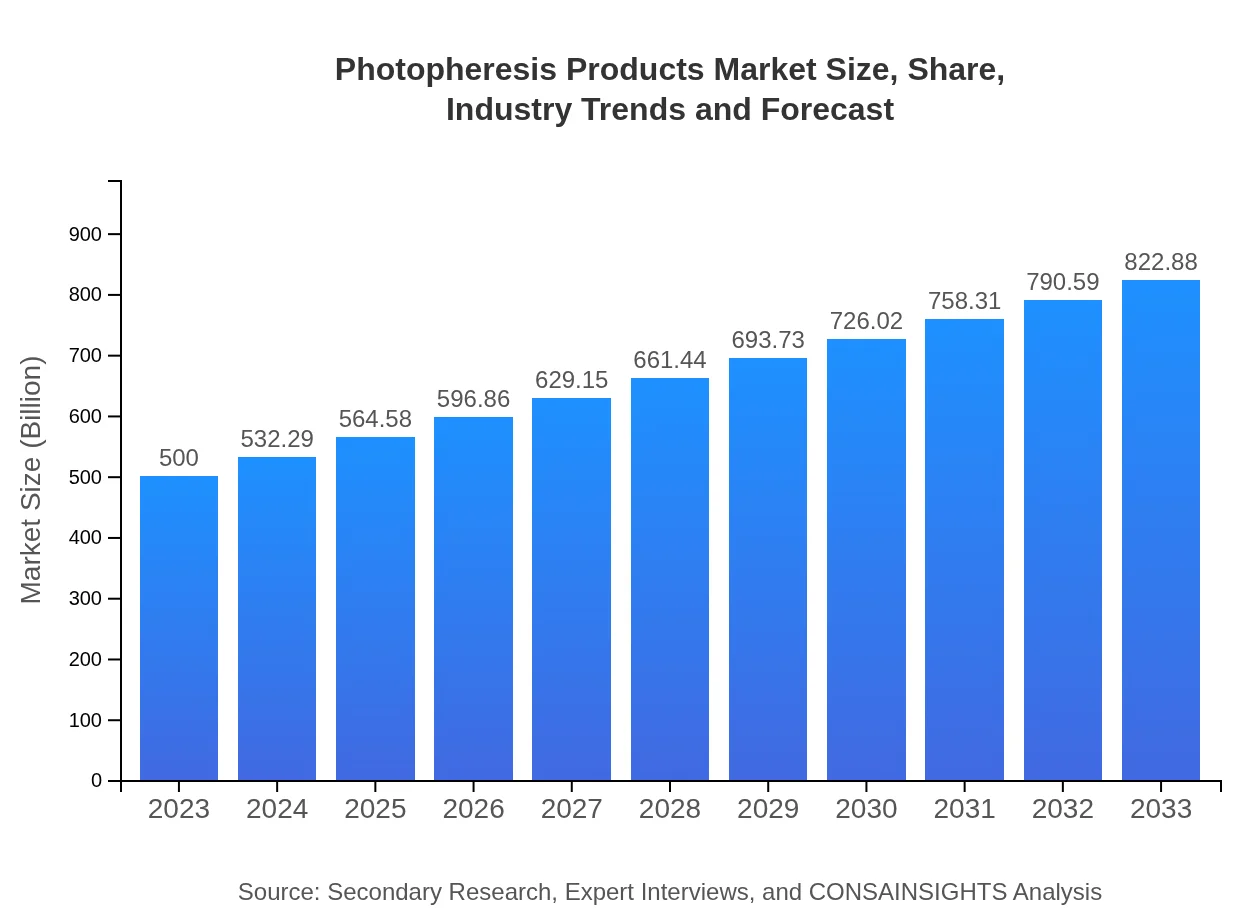
| 2023 Market Size | $500.00 Million |
| CAGR (2023-2033) | 5% |
| 2033 Market Size | $822.88 Million |
| Top Companies | Therakos, Inc., Fresenius Kabi AG, MediClonal |
| Last Modified Date | 31 January 2026 |
Photopheresis Products Market Overview
Customize Photopheresis Products Market Report market research report
- ✔ Get in-depth analysis of Photopheresis Products market size, growth, and forecasts.
- ✔ Understand Photopheresis Products's regional dynamics and industry-specific trends.
- ✔ Identify potential applications, end-user demand, and growth segments in Photopheresis Products
What is the Market Size & CAGR of Photopheresis Products market in 2023?
Photopheresis Products Industry Analysis
Photopheresis Products Market Segmentation and Scope
Tell us your focus area and get a customized research report.
Photopheresis Products Market Analysis Report by Region
Europe Photopheresis Products Market Report:
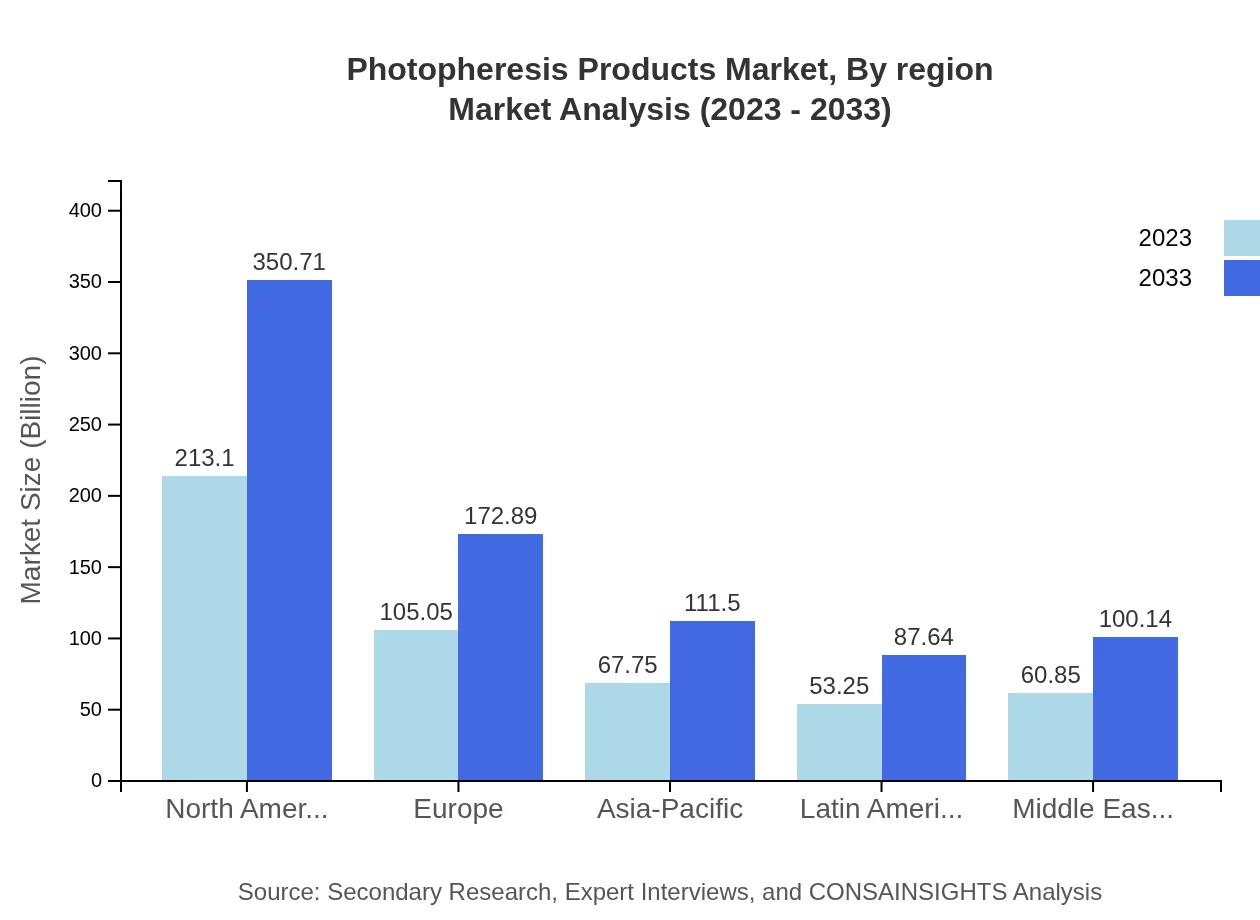
The European market is set to grow from USD 136.75 million in 2023 to USD 225.06 million by 2033. Key drivers include rising funding in research and development, alongside a focus on alternative therapeutic interventions to traditional treatments, supporting wider acceptance of photopheresis.Asia Pacific Photopheresis Products Market Report:
In 2023, the Photopheresis Products market in the Asia-Pacific region is estimated at USD 103.25 million, with an expected growth to USD 169.93 million by 2033. The region's growth is driven by increasing investments in healthcare infrastructure and a rising prevalence of skin diseases, coupled with greater accessibility to innovative therapies.North America Photopheresis Products Market Report:
North America dominates the Photopheresis Products market, with a valuation of USD 172.80 million in 2023, anticipated to reach USD 284.39 million by 2033. The high prevalence of chronic diseases, combined with advanced healthcare systems and favorable reimbursement policies, propels market growth in this region.South America Photopheresis Products Market Report:
The South American market for Photopheresis Products is projected to grow from USD 43.45 million in 2023 to USD 71.51 million by 2033. The expansion of healthcare facilities and advancements in treatment modalities are significant contributing factors, alongside increased public awareness regarding the benefits of photopheresis.Middle East & Africa Photopheresis Products Market Report:
The market size in the Middle East and Africa is expected to escalate from USD 43.75 million in 2023 to USD 72.00 million by 2033, facilitated by increasing healthcare investments and rising awareness of disease management.Tell us your focus area and get a customized research report.
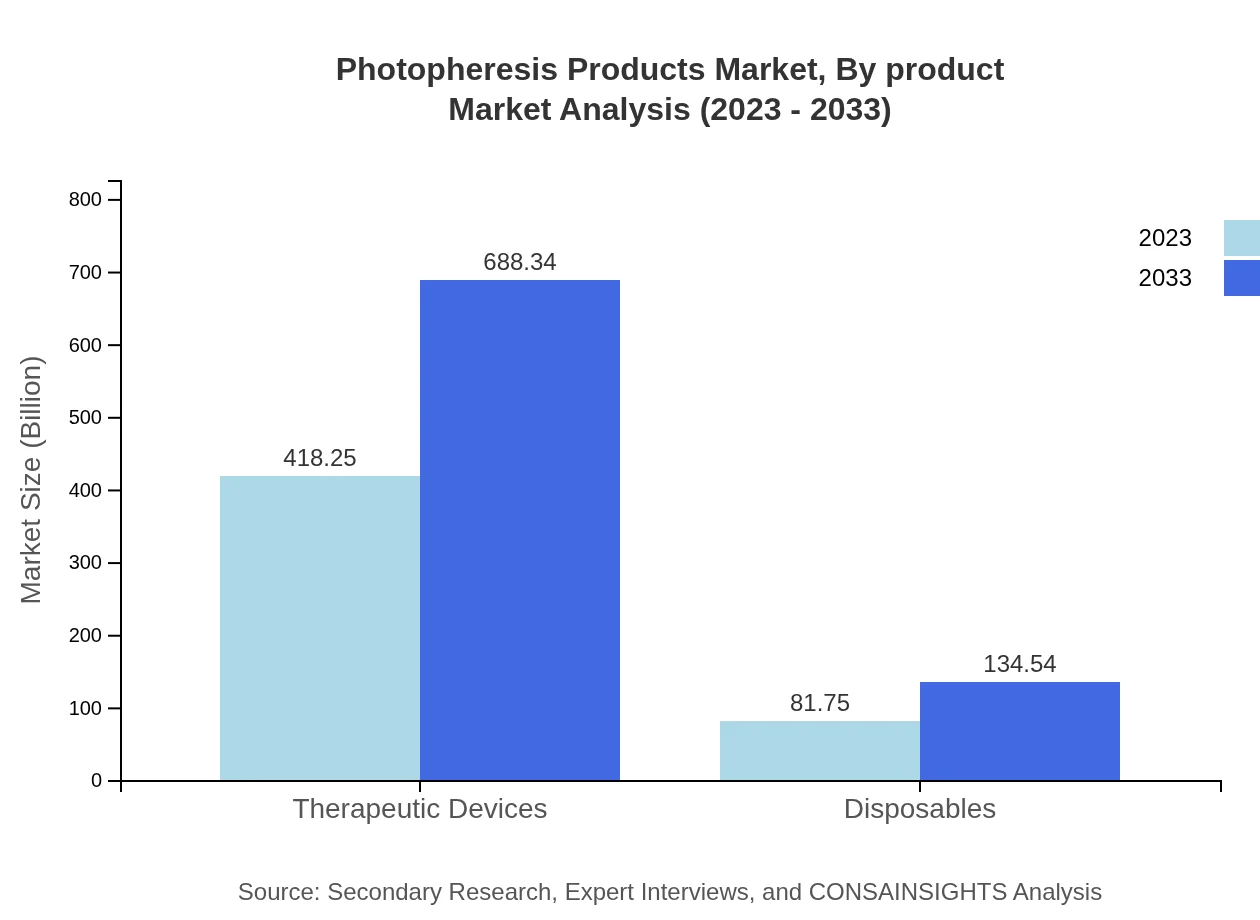
Photopheresis Products Market Analysis By Product
The Photopheresis Products market is segmented by product type into Therapeutic Devices and Disposables, where Therapeutic Devices account for a substantial share, comprising USD 418.25 million in 2023, growing to USD 688.34 million by 2033. Disposables segment is valued at USD 81.75 million in 2023, projected to reach USD 134.54 million by 2033.
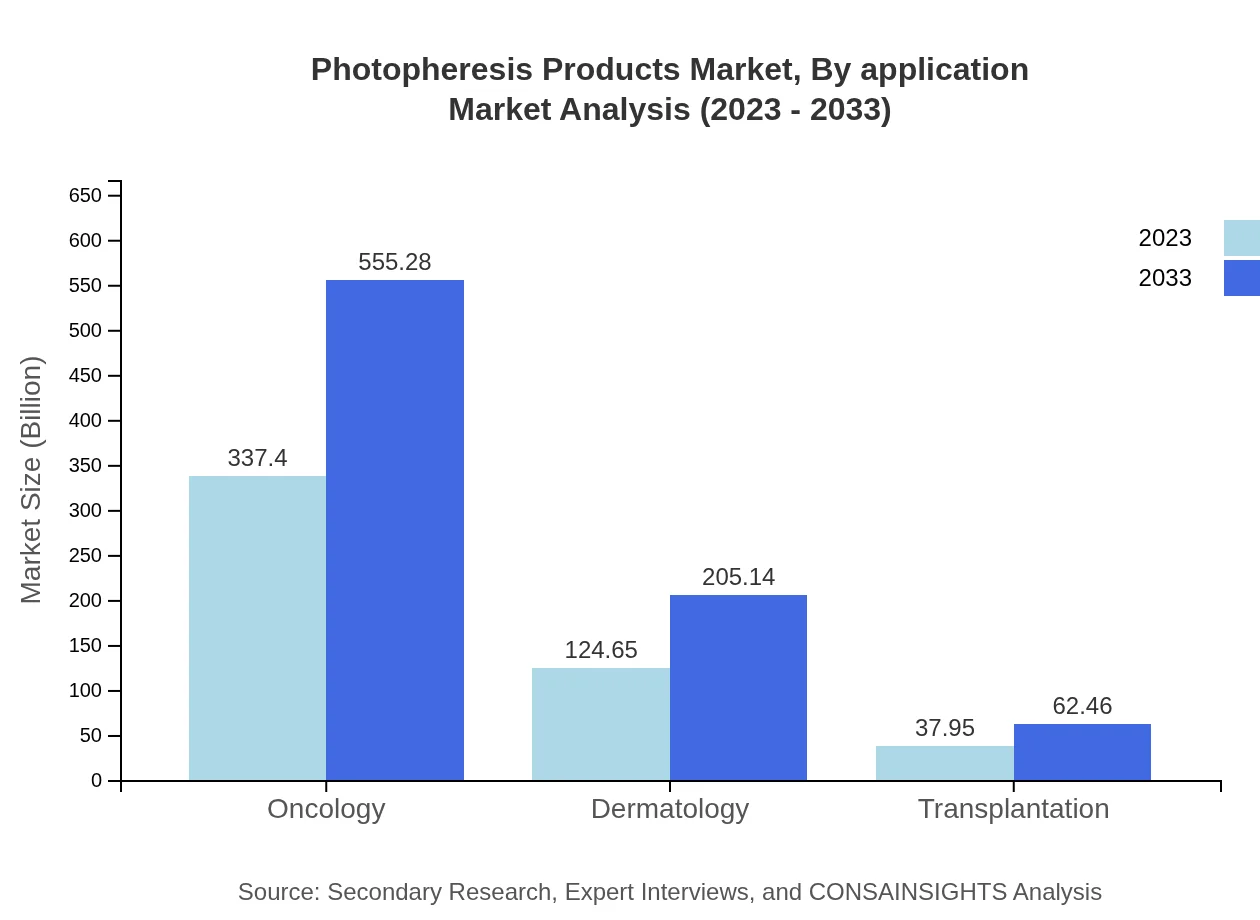
Photopheresis Products Market Analysis By Application
Applications of Photopheresis Products include Oncology, Dermatology, and Transplantation. Oncology holds a significant market share, valued at approximately USD 337.40 million in 2023, anticipated to reach USD 555.28 million by 2033. Dermatology and Transplantation follow, with projected growth rates reflecting increasing cases and treatment needs.
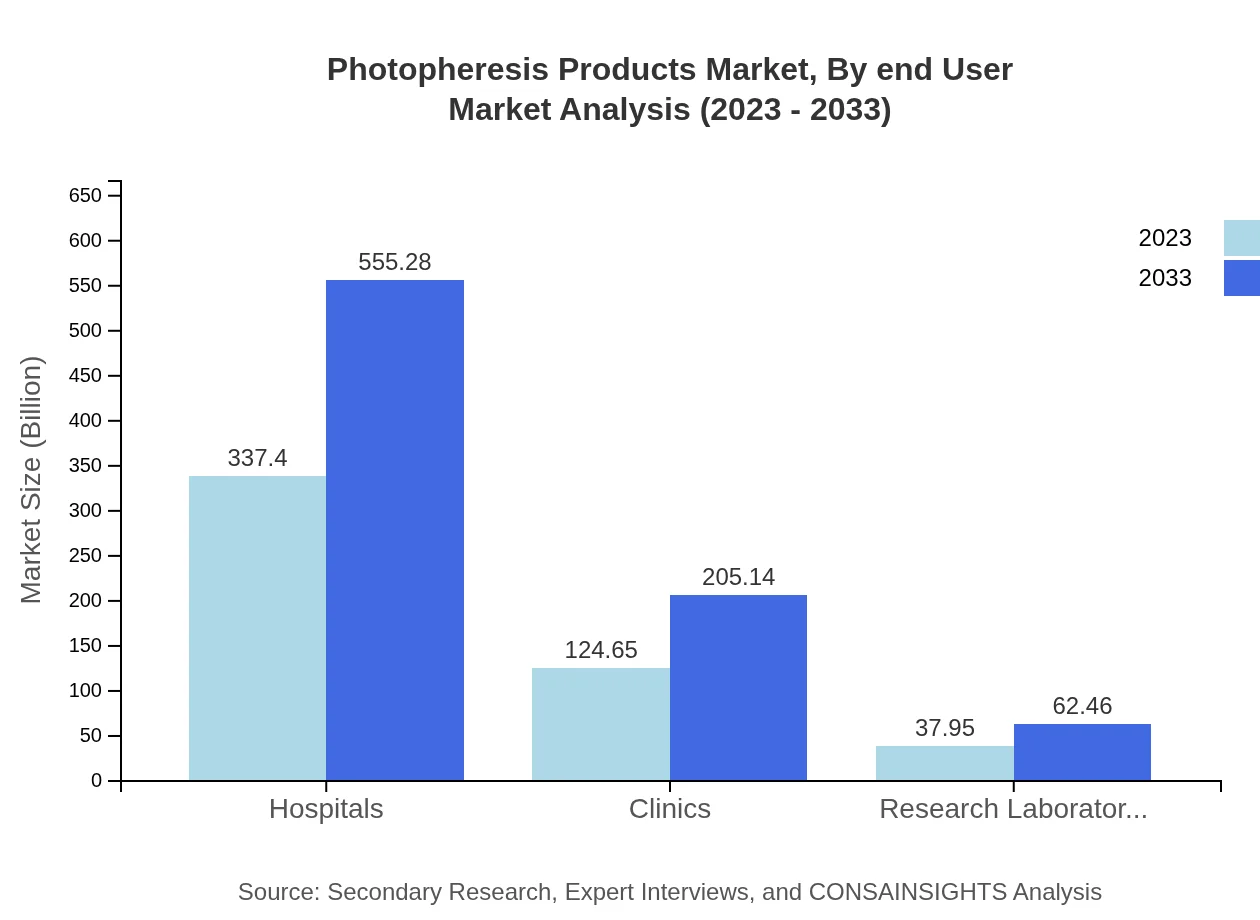
Photopheresis Products Market Analysis By End User
End-users include Hospitals, Clinics, and Research Laboratories. In 2023, Hospitals represent the largest segment with market values of USD 337.40 million, followed by Clinics at USD 124.65 million and Research Laboratories at USD 37.95 million. By 2033, these figures are expected to rise significantly across all segments.
Photopheresis Products Market Analysis By Region
Regionally, North America leads in market size, followed by Europe and Asia Pacific. The trends show significant growth in emerging markets in Asia Pacific and Latin America, indicating a shift in acceptance and accessibility to therapeutic interventions across these regions.
Photopheresis Products Market Trends and Future Forecast
Tell us your focus area and get a customized research report.
Global Market Leaders and Top Companies in Photopheresis Products Industry
Therakos, Inc.:
A prominent player specializing in photopheresis technology with a focus on innovation and improved patient care.Fresenius Kabi AG:
A leading global healthcare company specializing in lifesaving medicines and technologies, including photopheresis machines.MediClonal:
A biotechnology firm engaged in the development of new therapies utilizing photopheresis for various indications.We're grateful to work with incredible clients.









FAQs
What is the market size of photopheresis Products?
The global photopheresis products market is estimated to be worth approximately $500 million in 2023, with a projected CAGR of 5% from 2023 to 2033. This growth is indicative of increasing adoption in therapeutic applications.
What are the key market players or companies in this photopheresis Products industry?
Key players in the photopheresis products market include leading medical device companies specializing in therapeutic and diagnostic devices. These companies are at the forefront of innovation and technology advancements in the industry.
What are the primary factors driving the growth in the photopheresis Products industry?
Driving factors include an increased prevalence of chronic diseases, heightened awareness of photopheresis benefits, and advancements in technology and product design, which together enhance treatment efficacy and patient outcomes.
Which region is the fastest Growing in the photopheresis Products?
The fastest-growing region for photopheresis products is North America, expected to rise from $172.80 million in 2023 to $284.39 million by 2033. This growth can be attributed to robust healthcare infrastructure and rising disease incidences.
Does ConsaInsights provide customized market report data for the photopheresis Products industry?
Yes, ConsaInsights offers customized market report data tailored to specific needs within the photopheresis products industry, catering to unique client requirements and strategic insights.
What deliverables can I expect from this photopheresis Products market research project?
Deliverables include comprehensive market analysis reports, regional growth forecasts, competitive landscape assessments, segment data, and actionable recommendations based on in-depth research.
What are the market trends of photopheresis Products?
Current trends include an increasing focus on personalized medicine, technological innovations in photopheresis devices, expanding applications in oncology, and rising investments in research and development across the healthcare sector.