Plasma Protease C1 Inhibitor Market Report
Published Date: 31 January 2026 | Report Code: plasma-protease-c1-inhibitor
Plasma Protease C1 Inhibitor Market Size, Share, Industry Trends and Forecast to 2033
This report provides a comprehensive analysis of the Plasma Protease C1 Inhibitor market from 2023 to 2033, highlighting market size, growth trends, segmentation, and regional insights, alongside competitive landscape assessments and future forecasts.
| Metric | Value |
|---|---|
| Study Period | 2023 - 2033 |
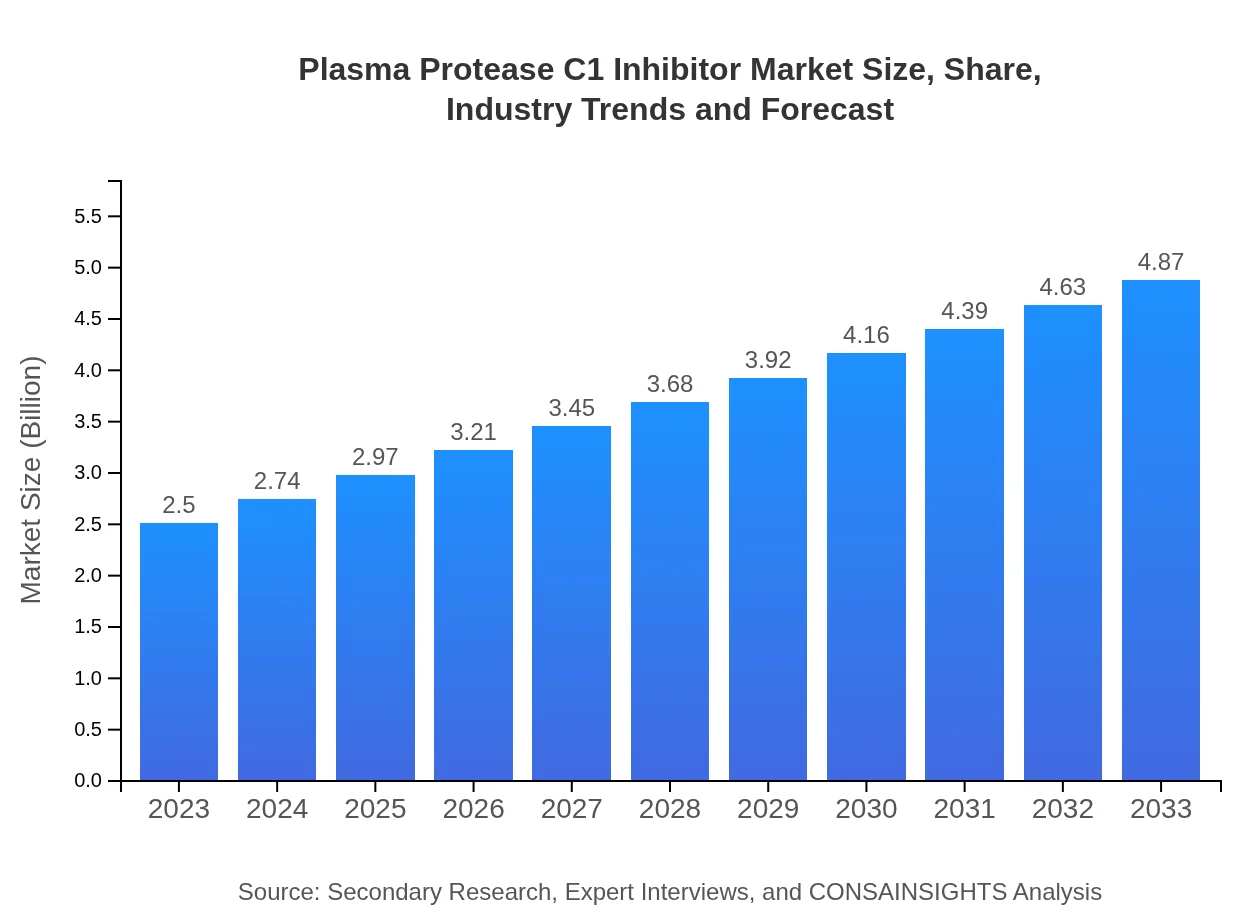
| 2023 Market Size | $2.50 Billion |
| CAGR (2023-2033) | 6.7% |
| 2033 Market Size | $4.87 Billion |
| Top Companies | Shire Pharmaceuticals, CSL Behring, Takeda Pharmaceuticals, Octapharma AG |
| Last Modified Date | 31 January 2026 |
Plasma Protease C1 Inhibitor Market Overview
Customize Plasma Protease C1 Inhibitor Market Report market research report
- ✔ Get in-depth analysis of Plasma Protease C1 Inhibitor market size, growth, and forecasts.
- ✔ Understand Plasma Protease C1 Inhibitor's regional dynamics and industry-specific trends.
- ✔ Identify potential applications, end-user demand, and growth segments in Plasma Protease C1 Inhibitor
What is the Market Size & CAGR of Plasma Protease C1 Inhibitor market in 2033?
Plasma Protease C1 Inhibitor Industry Analysis
Plasma Protease C1 Inhibitor Market Segmentation and Scope
Tell us your focus area and get a customized research report.
Plasma Protease C1 Inhibitor Market Analysis Report by Region
Europe Plasma Protease C1 Inhibitor Market Report:
Europe's market is foreseen to grow steadily, reaching $1.60 billion by 2033 from $0.82 billion in 2023. The presence of advanced healthcare systems, along with significant pharmaceutical firms, plays a critical role in this growth trajectory.Asia Pacific Plasma Protease C1 Inhibitor Market Report:
The Asia-Pacific region is anticipated to exhibit robust growth, driven by rising healthcare expenditures and increasing cases of hereditary angioedema. By 2033, the market size in this region is projected to reach approximately $0.91 billion, up from $0.47 billion in 2023, marking a substantial growth opportunity.North America Plasma Protease C1 Inhibitor Market Report:
North America maintains its dominance in the global Plasma Protease C1 Inhibitor market, with an expected market size of $1.69 billion by 2033, rising from $0.87 billion in 2023. Factors contributing to this growth include high prevalence rates of hereditary angioedema and robust healthcare infrastructure.South America Plasma Protease C1 Inhibitor Market Report:
In South America, market growth is propelled by increasing awareness and acceptance of advanced therapeutic options. The market size is expected to grow from $0.13 billion in 2023 to $0.24 billion in 2033, reflecting an increased penetration of modern healthcare practices.Middle East & Africa Plasma Protease C1 Inhibitor Market Report:
In the Middle East and Africa, growing healthcare initiatives and improved access to treatment options are anticipated to push market growth. The projected market size will rise from $0.22 billion in 2023 to $0.42 billion by 2033, indicating a burgeoning demand for these inhibitors.Tell us your focus area and get a customized research report.
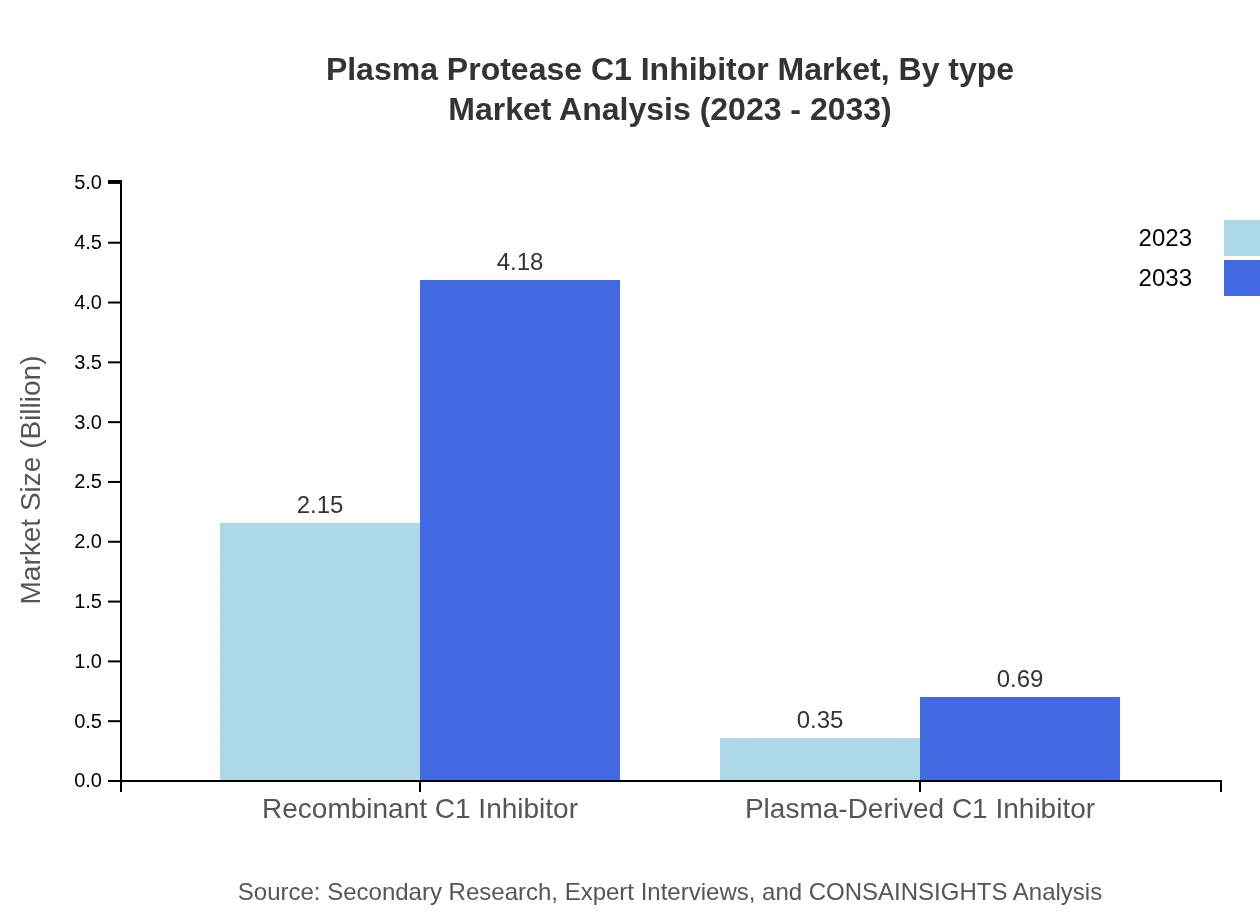
Plasma Protease C1 Inhibitor Market Analysis By Type
The Plasma Protease C1 Inhibitor market is primarily divided into two types: Recombinant C1 Inhibitor and Plasma-Derived C1 Inhibitor. The Recombinant C1 Inhibitor segment is forecasted to grow from $2.15 billion in 2023 to $4.18 billion by 2033, showcasing a dominant market share of 85.92% throughout the forecasted period. In contrast, the Plasma-Derived C1 Inhibitor, while crucial, represents a smaller segment with anticipated growth from $0.35 billion to $0.69 billion.
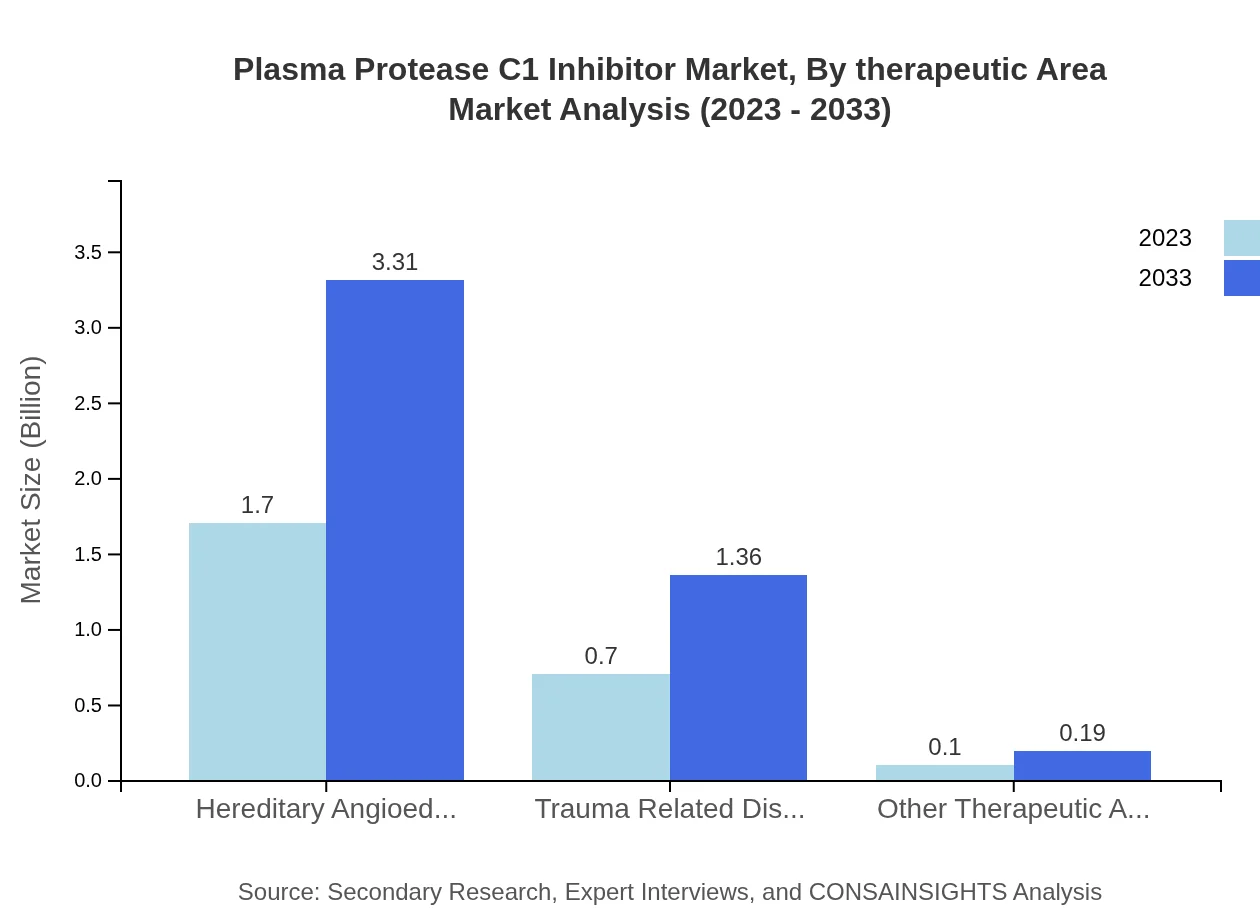
Plasma Protease C1 Inhibitor Market Analysis By Therapeutic Area
In terms of therapeutic areas, Hereditary Angioedema is the primary focus, representing a market size of $1.70 billion in 2023, projected to grow to $3.31 billion by 2033. Trauma related disorders are also gaining traction, expected to rise from $0.70 billion to $1.36 billion over the same period.
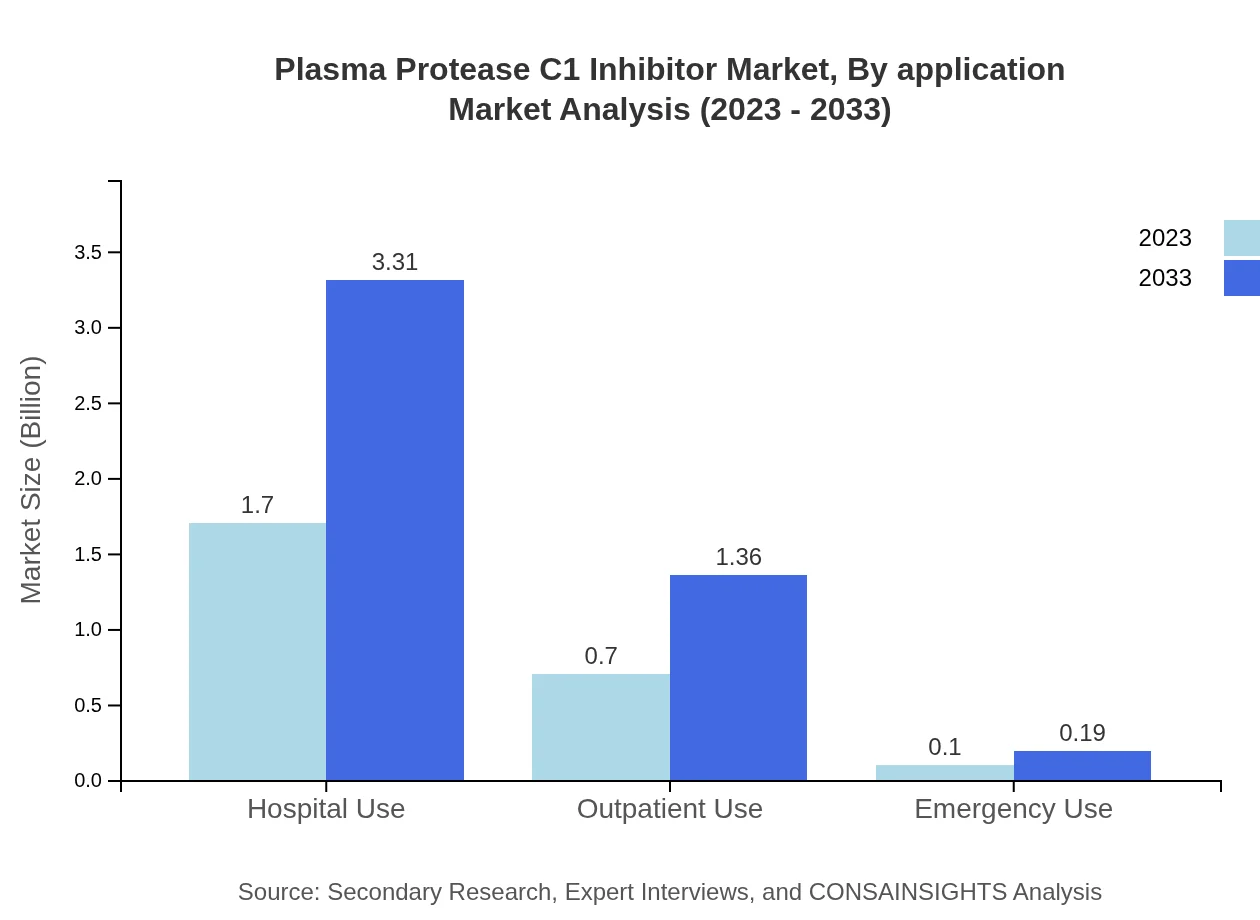
Plasma Protease C1 Inhibitor Market Analysis By Application
The application segment is categorized into Hospital Use, Outpatient Use, and Emergency Use. Hospital use holds the largest market share, growing from $1.70 billion in 2023 to $3.31 billion in 2033. Outpatient use is projected to rise from $0.70 billion to $1.36 billion, driven by an increasing trend toward managing conditions outside of hospital settings.
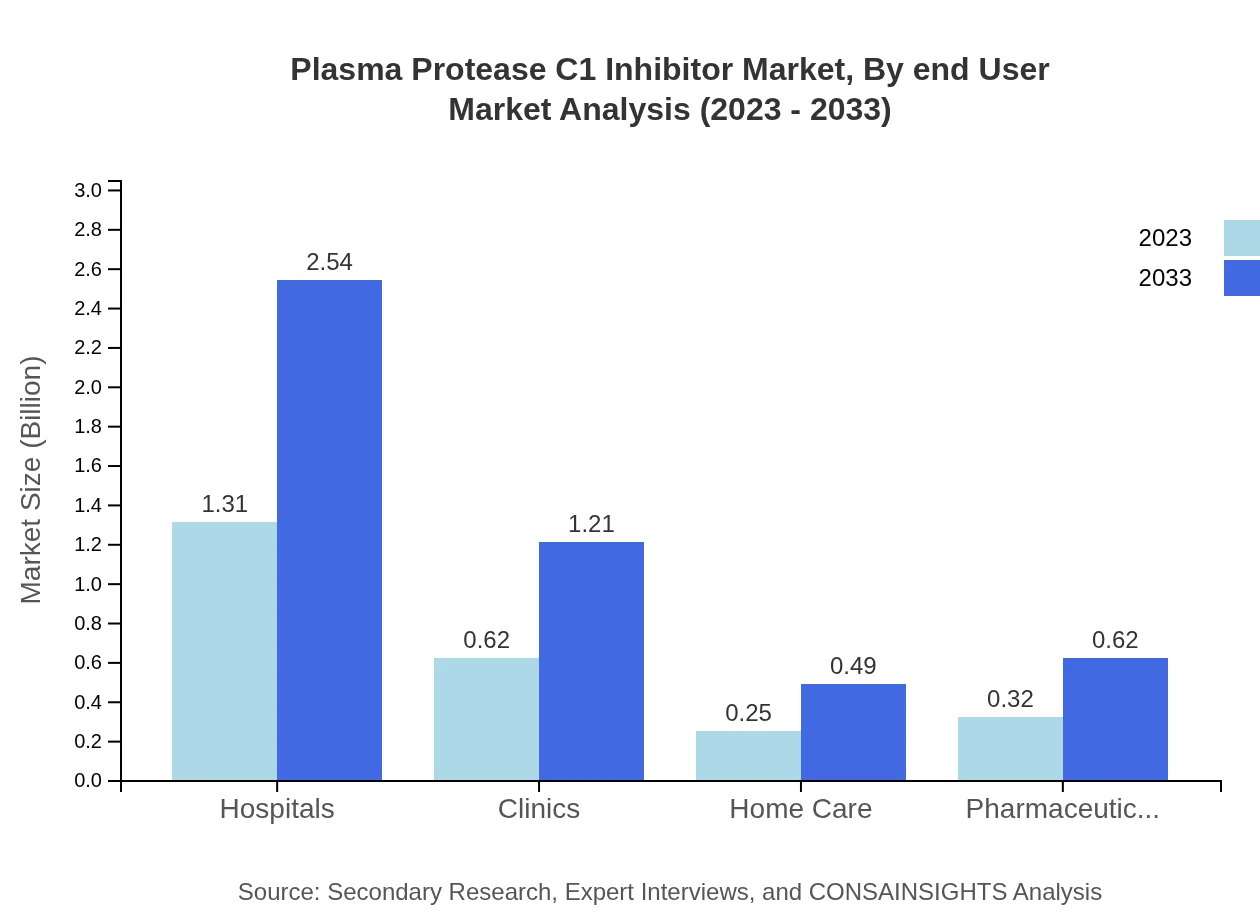
Plasma Protease C1 Inhibitor Market Analysis By End User
Major end-users of Plasma Protease C1 Inhibitors include Hospitals, Clinics, Home Care, and Pharmaceutical Companies. Hospitals dominate the market with a size expected to expand from $1.31 billion in 2023 to $2.54 billion by 2033, constituting 52.21% of the end-user market share.
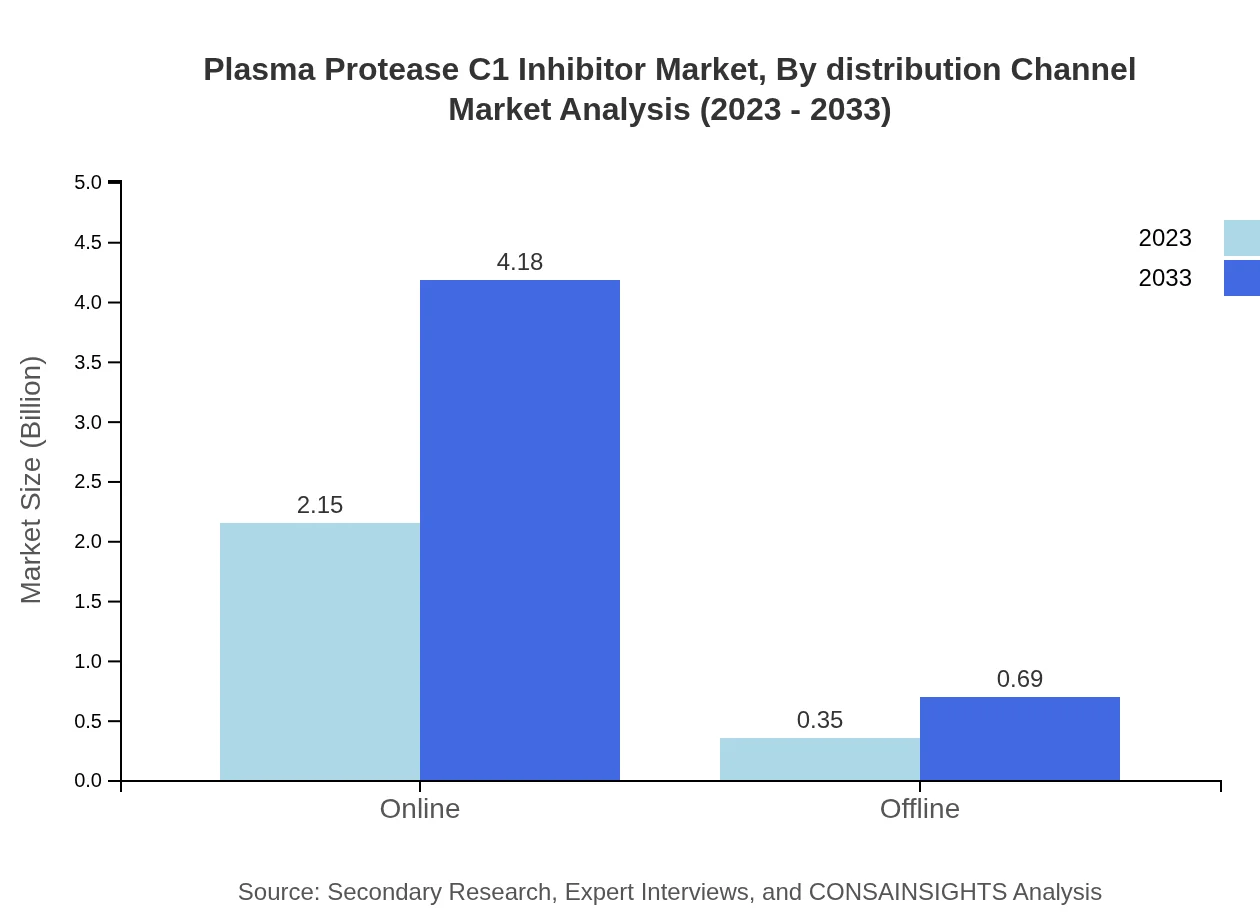
Plasma Protease C1 Inhibitor Market Analysis By Distribution Channel
Distribution channels for C1 inhibitors are segmented into Online and Offline. The online distribution channel is projected to grow significantly, anticipated to rise from $2.15 billion in 2023 to $4.18 billion by 2033, capturing 85.92% of the overall distribution market share, reflecting shifting buying preferences toward digital platforms.
Plasma Protease C1 Inhibitor Market Trends and Future Forecast
Tell us your focus area and get a customized research report.
Global Market Leaders and Top Companies in Plasma Protease C1 Inhibitor Industry
Shire Pharmaceuticals:
A pioneer in therapies for rare diseases, Shire develops various recombinant and plasma-derived products for managing hereditary angioedema.CSL Behring:
Known for its extensive range of therapies, CSL Behring specializes in plasma-derived products, including Plasma Protease C1 Inhibitor, contributing significantly to global healthcare.Takeda Pharmaceuticals:
Takeda is involved in the development and commercialization of innovative therapies, focusing on hereditary angioedema treatments and their advancements.Octapharma AG:
A global leader in the development of human proteins from human plasma, Octapharma offers vital treatments for patients with severe hereditary angioedema.We're grateful to work with incredible clients.









FAQs
What is the market size of Plasma Protease C1 Inhibitor?
The Plasma Protease C1 Inhibitor market was valued at approximately $2.5 billion in 2023, with a projected CAGR of 6.7% over the next decade, indicating strong growth potential driven by advancements in therapies and increasing awareness.
What are the key market players or companies in this Plasma Protease C1 Inhibitor industry?
Key players in the Plasma Protease C1 Inhibitor market include companies like CSL Behring, Shire, and Grifols. These companies lead in innovation and supply, contributing significantly to market growth by developing new treatments and expanding their product offerings.
What are the primary factors driving the growth in the Plasma Protease C1 Inhibitor industry?
Growth in the Plasma Protease C1 Inhibitor market is driven by an increase in diagnoses of hereditary angioedema, advancements in recombinant therapy development, and rising healthcare expenditures focused on rare diseases, which enhance treatment accessibility and affordability.
Which region is the fastest Growing in the Plasma Protease C1 Inhibitor?
The fastest-growing region in the Plasma Protease C1 Inhibitor market is North America, projected to grow from $0.87 billion in 2023 to $1.69 billion by 2033. This growth is driven by high healthcare spending and increasing awareness of treatment options.
Does ConsaInsights provide customized market report data for the Plasma Protease C1 Inhibitor industry?
Yes, ConsaInsights offers customized market report data tailored to specific needs in the Plasma Protease C1 Inhibitor industry, including detailed analysis by segment, region, and competitive landscape to suit unique business strategies.
What deliverables can I expect from this Plasma Protease C1 Inhibitor market research project?
From this project, expect comprehensive deliverables including market size estimates, growth forecasts, competitive analysis, regional insights, and detailed segment breakdowns addressing both current trends and future growth opportunities.
What are the market trends of Plasma Protease C1 Inhibitor?
Key trends in the Plasma Protease C1 Inhibitor market include a shift towards recombinant therapies, increasing adoption in hospitals and outpatient settings, and a growing focus on patient access programs, driving a more personalized approach to treatment.