Plasma Protein Therapeutics Market Report
Published Date: 31 January 2026 | Report Code: plasma-protein-therapeutics
Plasma Protein Therapeutics Market Size, Share, Industry Trends and Forecast to 2033
This report comprehensively analyzes the Plasma Protein Therapeutics market, highlighting key trends, growth forecasts, and detailed insights across different regions and segments from 2023 to 2033.
| Metric | Value |
|---|---|
| Study Period | 2023 - 2033 |
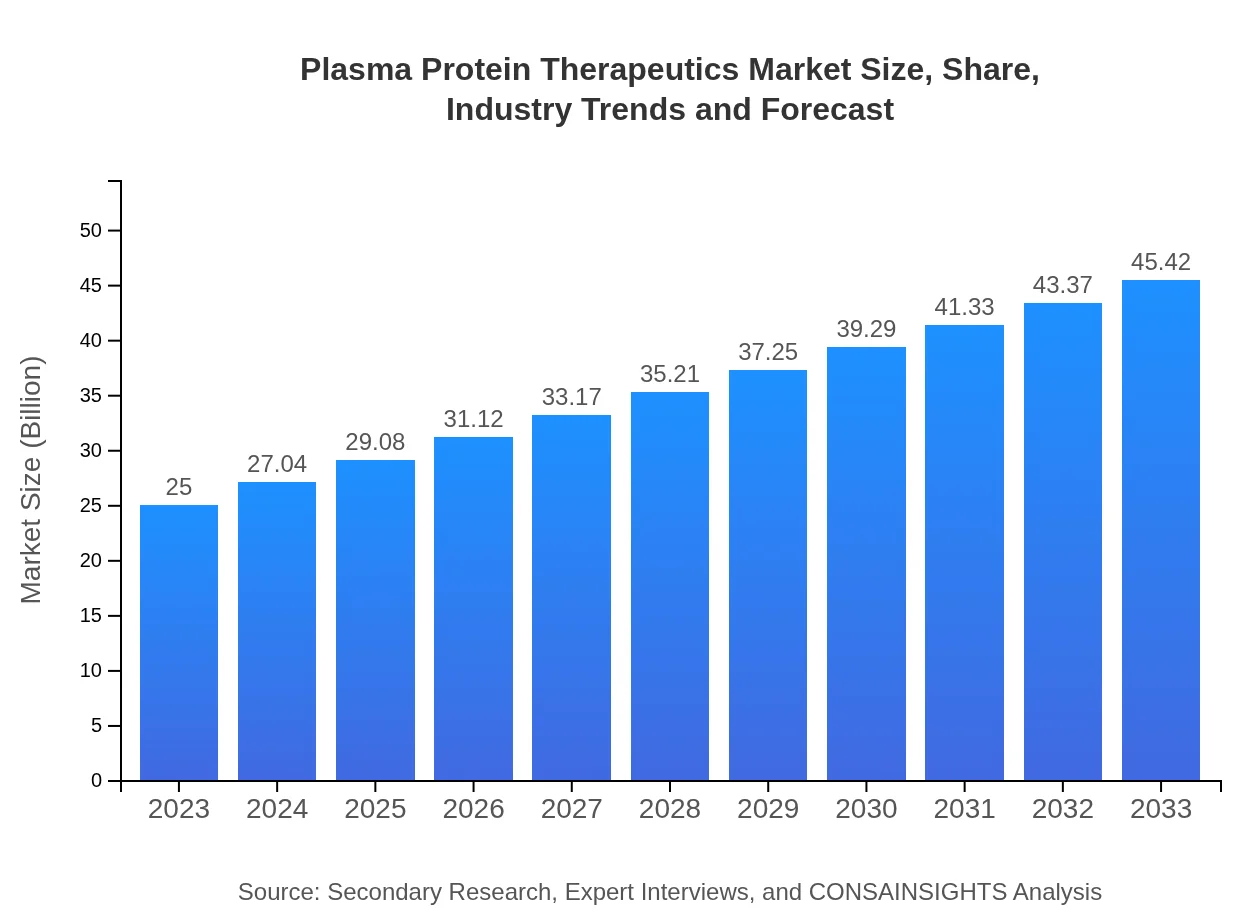
| 2023 Market Size | $25.00 Billion |
| CAGR (2023-2033) | 6% |
| 2033 Market Size | $45.42 Billion |
| Top Companies | Grifols, CSL Behring, Takeda Pharmaceutical Company, AbbVie, Octapharma |
| Last Modified Date | 31 January 2026 |
Plasma Protein Therapeutics Market Overview
Customize Plasma Protein Therapeutics Market Report market research report
- ✔ Get in-depth analysis of Plasma Protein Therapeutics market size, growth, and forecasts.
- ✔ Understand Plasma Protein Therapeutics's regional dynamics and industry-specific trends.
- ✔ Identify potential applications, end-user demand, and growth segments in Plasma Protein Therapeutics
What is the Market Size & CAGR of Plasma Protein Therapeutics market in 2023?
Plasma Protein Therapeutics Industry Analysis
Plasma Protein Therapeutics Market Segmentation and Scope
Tell us your focus area and get a customized research report.
Plasma Protein Therapeutics Market Analysis Report by Region
Europe Plasma Protein Therapeutics Market Report:
The European market is expected to see significant growth from USD 8.26 billion in 2023 to USD 15.00 billion by 2033. Factors such as older population demographics and extensive research initiatives are paving the way for new therapeutic solutions.Asia Pacific Plasma Protein Therapeutics Market Report:
In the Asia Pacific region, the Plasma Protein Therapeutics market is expected to grow from USD 4.21 billion in 2023 to USD 7.65 billion by 2033. The increasing incidence of chronic diseases and rising healthcare expenditure are key drivers of growth. Regional players are also focusing on establishing partnerships and expanding distribution networks to enhance market penetration.North America Plasma Protein Therapeutics Market Report:
North America leads the market with a projected growth from USD 9.03 billion in 2023 to USD 16.40 billion by 2033. A robust healthcare system, high prevalence of target diseases, and the presence of major companies underline this growth trajectory.South America Plasma Protein Therapeutics Market Report:
The South American market, though relatively smaller, is anticipated to grow from USD 0.69 billion in 2023 to USD 1.25 billion by 2033. Increased awareness regarding plasma therapies and improved healthcare infrastructure are elements contributing to market expansion in this region.Middle East & Africa Plasma Protein Therapeutics Market Report:
The Middle East and Africa market is anticipated to grow from USD 2.81 billion in 2023 to USD 5.11 billion by 2033. Improving healthcare infrastructure and the growing prevalence of bleeding disorders are driving demand in this region.Tell us your focus area and get a customized research report.
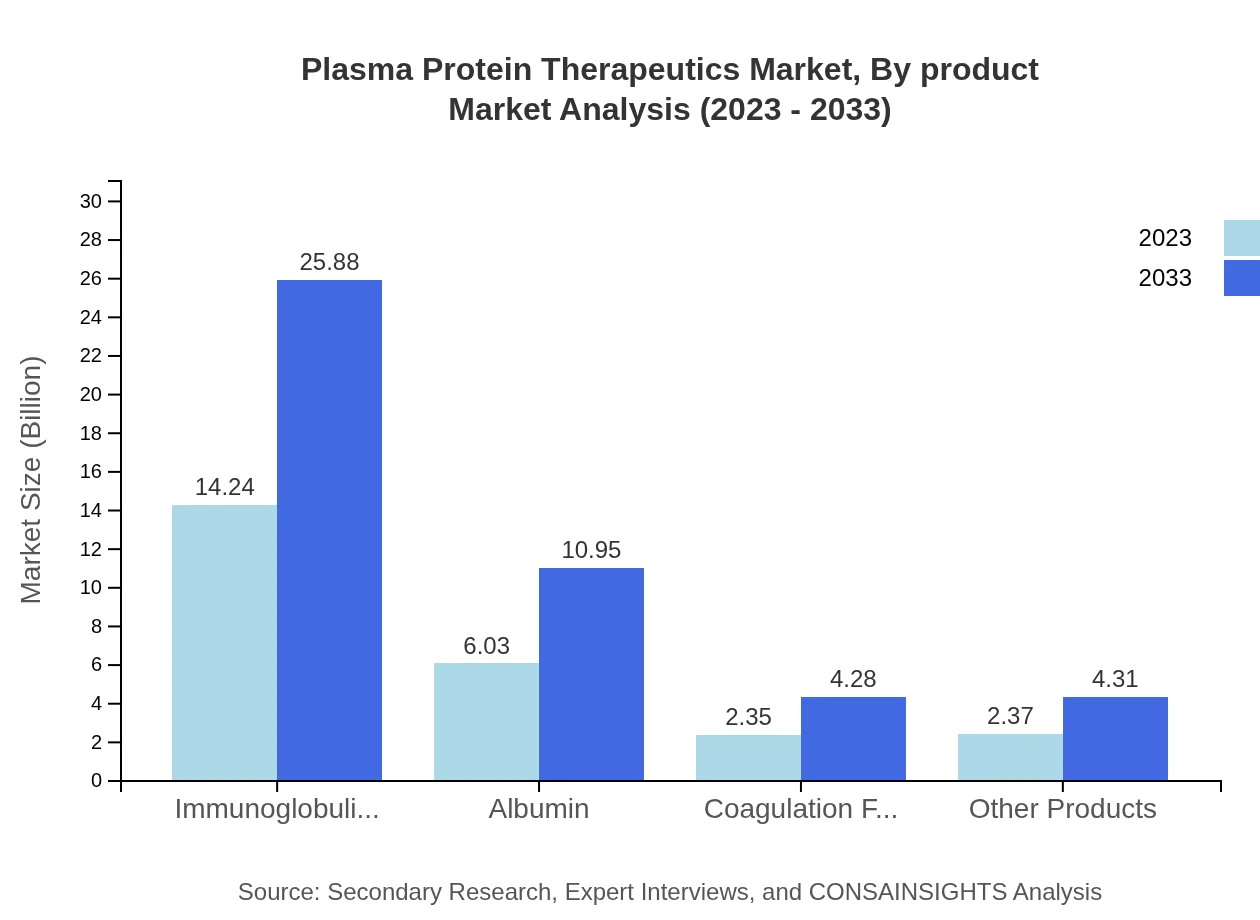
Plasma Protein Therapeutics Market Analysis By Product
The product segmentation reveals that immunoglobulins dominate the market, expected to total USD 25.88 billion in size by 2033, holding around 56.98% market share. Albumin follows, projected to reach USD 10.95 billion representing 24.12% of the market share. Coagulation factors and other products contribute significantly, underscoring the diverse therapeutic potentials of plasma protein therapies.
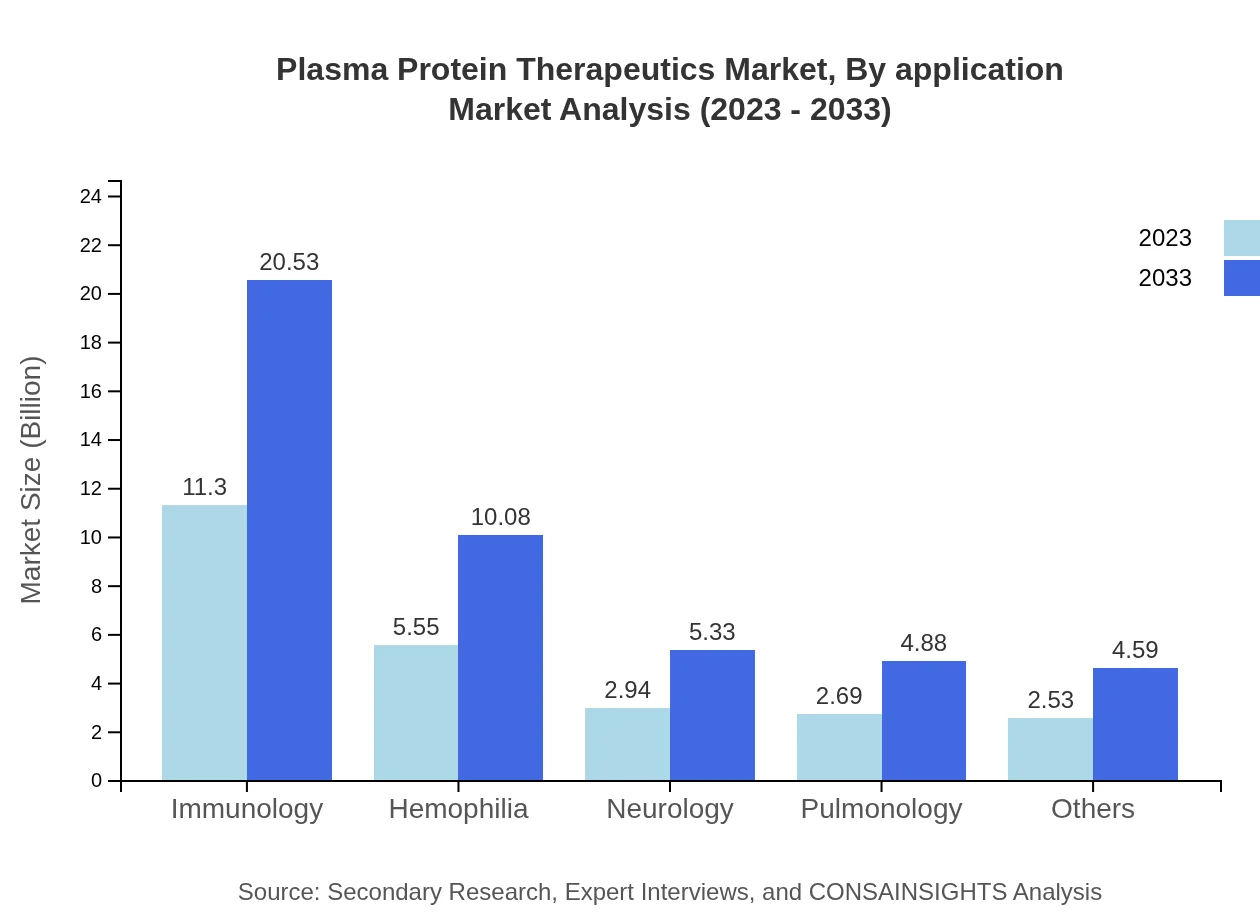
Plasma Protein Therapeutics Market Analysis By Application
In terms of application, the immunology segment leads with expected revenues of USD 20.53 billion by 2033, comprising 45.21% of the market share. Hemophilia represents a major segment as well, with estimations of USD 10.08 billion. This indicates that treatment for chronic conditions is a key focus area in the plasma protein market.
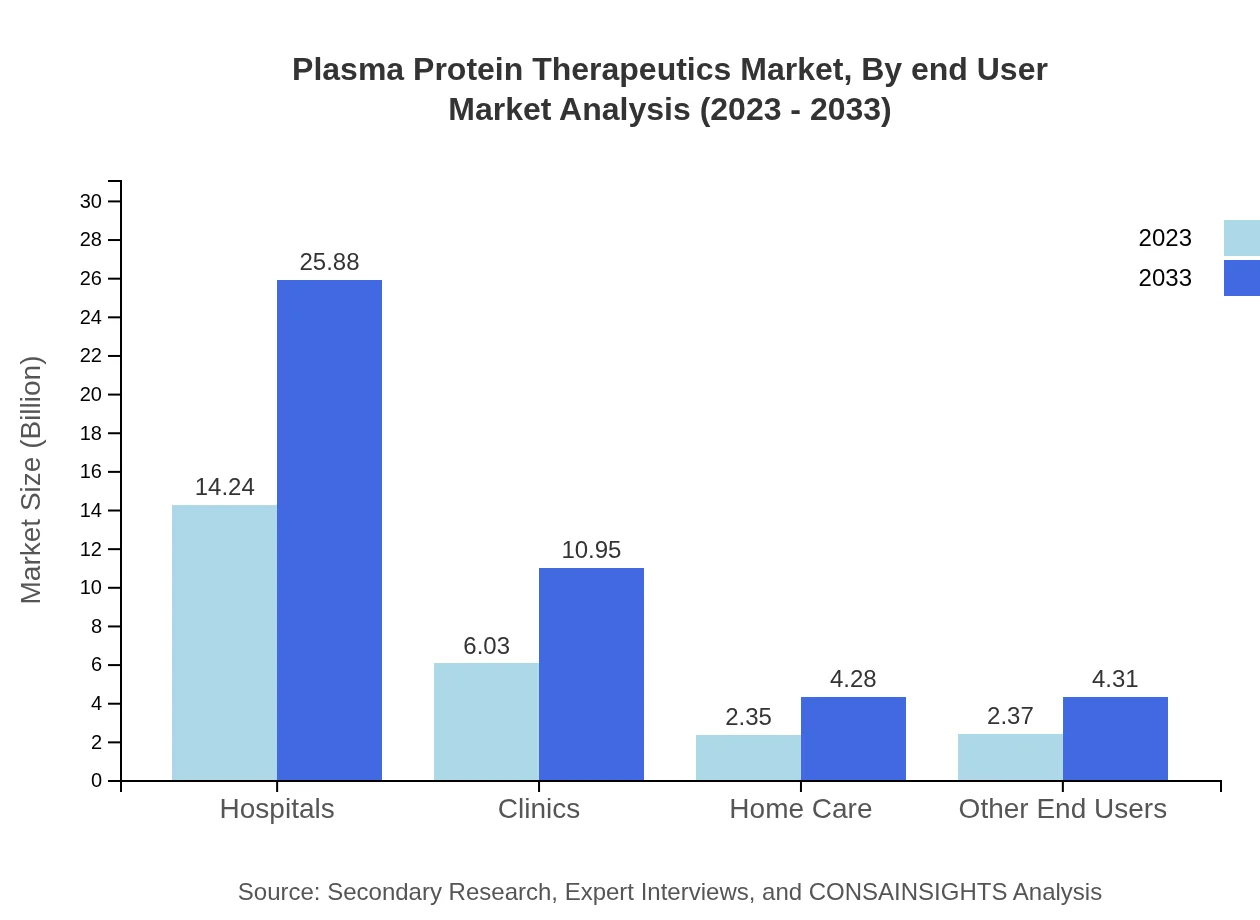
Plasma Protein Therapeutics Market Analysis By End User
Hospitals, as the primary end-user, are projected to hold USD 25.88 billion market share by 2033 (56.98%). Clinics and home care settings will also see increased adoption, highlighting shifts in patient treatment modalities and care settings in the plasma therapeutics landscape.
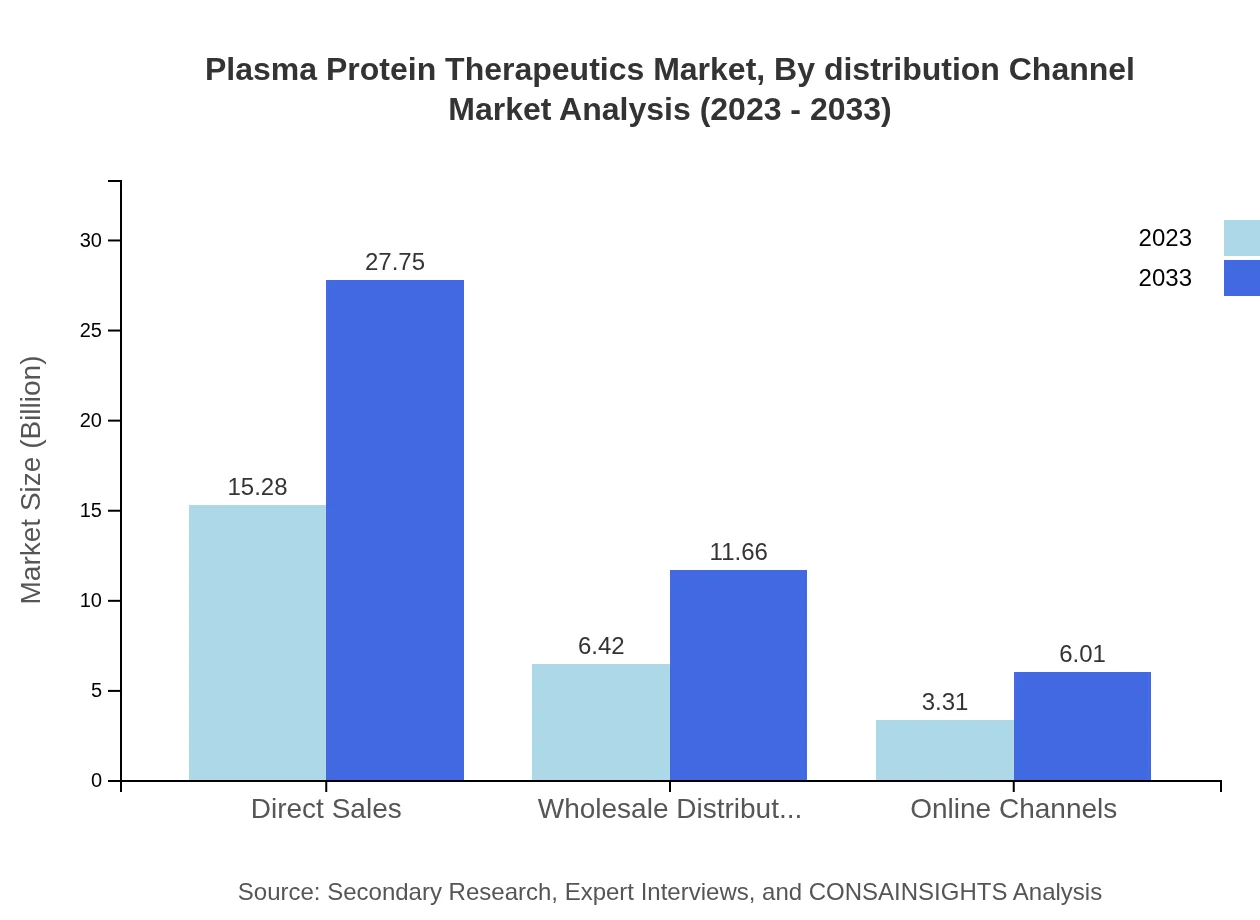
Plasma Protein Therapeutics Market Analysis By Distribution Channel
The distribution channels segment reflects a dominance of direct sales, representing 61.1% of market share. Emerging channels such as online sales are also on the rise, showcasing the evolution of how therapies are accessed by healthcare providers and patients alike.
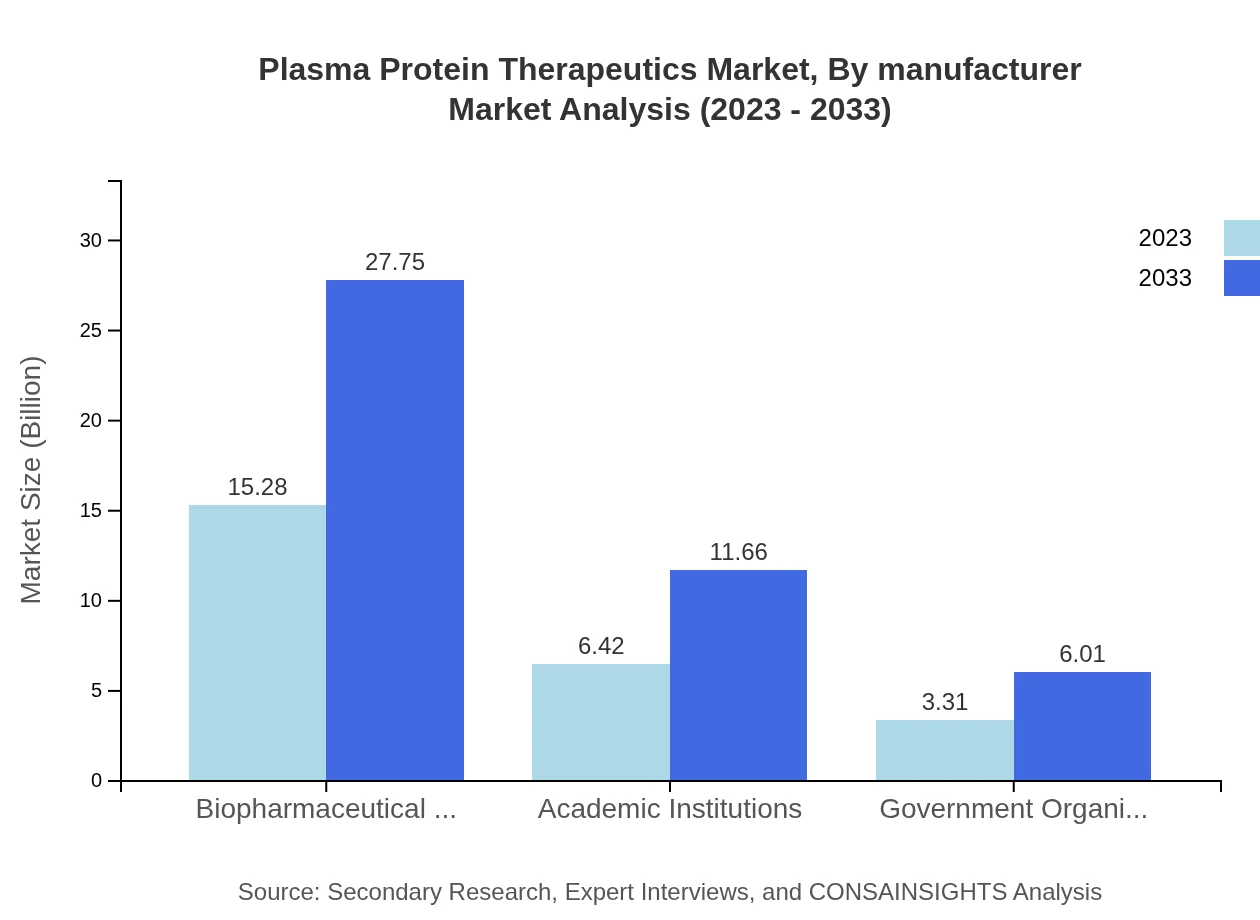
Plasma Protein Therapeutics Market Analysis By Manufacturer
Biopharmaceutical companies dominate the manufacturer segment with approximately 61.1% of the market share. Their focus on innovation and expansion into diverse therapeutic areas is crucial for continued growth in the Plasma Protein Therapeutics sector.
Plasma Protein Therapeutics Market Trends and Future Forecast
Tell us your focus area and get a customized research report.
Global Market Leaders and Top Companies in Plasma Protein Therapeutics Industry
Grifols:
A global healthcare company specializing in the production of plasma-derived medicines, Grifols leads the industry in innovation and research initiatives.CSL Behring:
Recognized for its extensive range of therapies derived from human plasma, CSL Behring maintains a strong market presence through continual product development and outreach.Takeda Pharmaceutical Company:
With a focus on transformative therapies for patients, Takeda invests heavily in R&D, enhancing its portfolio in plasma therapeutics.AbbVie:
A major player with extensive offerings in immunology, AbbVie is instrumental in expanding treatment options for patients with plasma-derived therapies.Octapharma:
Known for its high-quality plasmaderived therapies, Octapharma emphasizes innovation and patient-centered care in its approach.We're grateful to work with incredible clients.









FAQs
What is the market size of plasma Protein Therapeutics?
The plasma-protein therapeutics market is valued at approximately $25 billion in 2023, with a projected CAGR of 6% through 2033. This indicates a sustained interest and expansion in the therapeutic applications derived from plasma proteins.
What are the key market players or companies in this plasma Protein Therapeutics industry?
Major players in the plasma-protein therapeutics market include biopharmaceutical companies and relevant academic institutions. These entities play pivotal roles in innovation, research, and distribution of therapeutic products derived from plasma proteins.
What are the primary factors driving the growth in the plasma Protein Therapeutics industry?
Growth in this market is driven by increased demand for innovative therapies, advancements in technology, and a rising prevalence of diseases treatable by plasma proteins. The expanding healthcare sectors in developing nations also contribute significantly to this growth.
Which region is the fastest Growing in the plasma Protein Therapeutics?
North America leads the fastest growth in the plasma-protein therapeutics market, with a value projected to rise from $9.03 billion in 2023 to $16.40 billion by 2033. However, Europe also shows significant potential with comparable growth.
Does ConsaInsights provide customized market report data for the plasma Protein Therapeutics industry?
Yes, ConsaInsights offers tailored market report data for the plasma-protein therapeutics industry, allowing clients to obtain specific insights and analysis that cater to their strategic needs and market objectives.
What deliverables can I expect from this plasma Protein Therapeutics market research project?
Deliverables typically include a detailed market report highlighting market size, growth rates, trends, regional analysis, and insights into competitive dynamics among key players in the plasma-protein therapeutics industry.
What are the market trends of plasma Protein Therapeutics?
Current trends indicate an increasing focus on treatment personalization, growth in home care applications, and innovation in therapeutic products. Segment growth, particularly in immunoglobulins, is also notable, expected to expand significantly by 2033.