Pneumococcal Vaccines Market Report
Published Date: 31 January 2026 | Report Code: pneumococcal-vaccines
Pneumococcal Vaccines Market Size, Share, Industry Trends and Forecast to 2033
This report provides a comprehensive analysis of the Pneumococcal Vaccines market from 2023 to 2033, including market size, growth trends, regional insights, technological advancements, and competitive landscape. Insights into market segmentation, leading companies, and future forecasts are included to guide stakeholders.
| Metric | Value |
|---|---|
| Study Period | 2023 - 2033 |
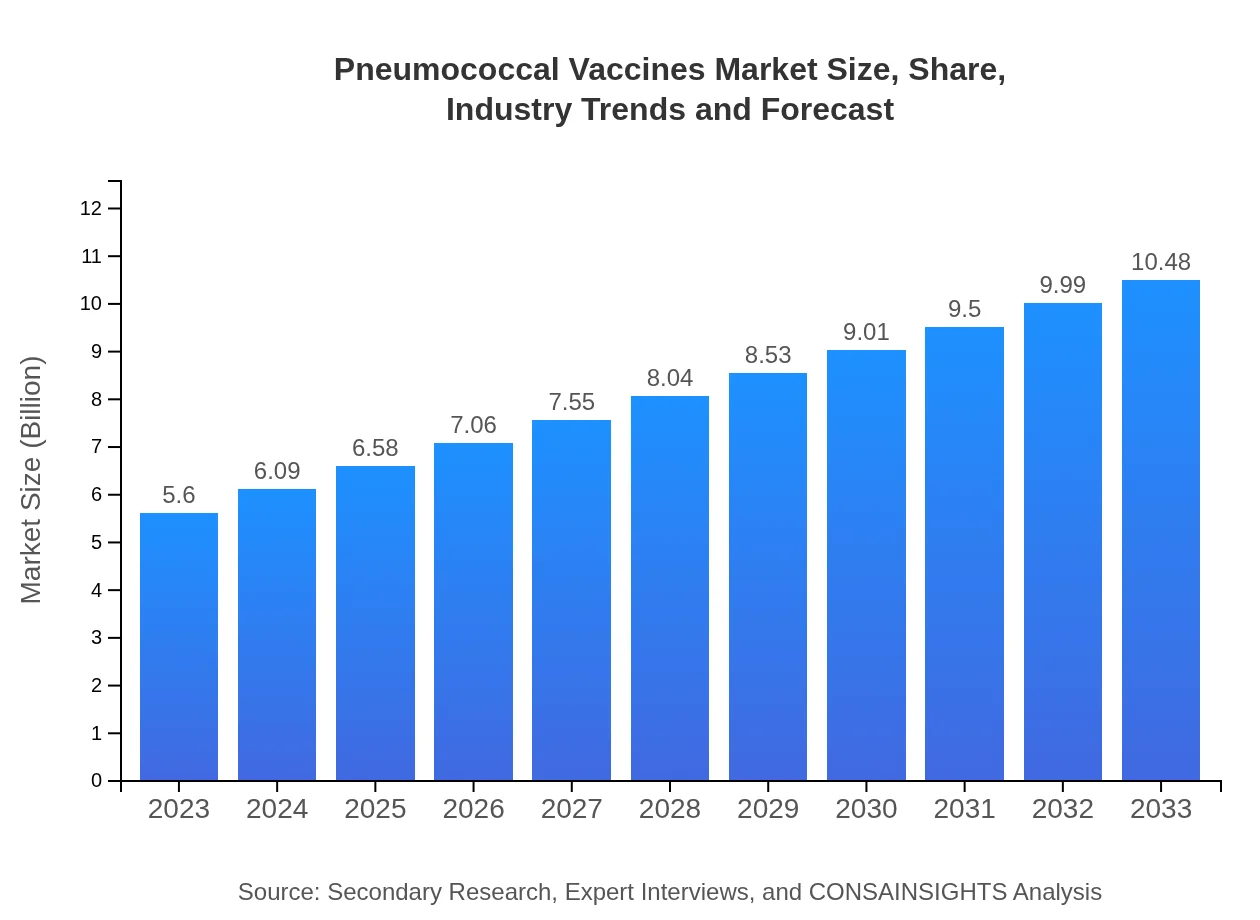
| 2023 Market Size | $5.60 Billion |
| CAGR (2023-2033) | 6.3% |
| 2033 Market Size | $10.48 Billion |
| Top Companies | Pfizer Inc., Merck & Co., Inc., Sanofi Pasteur, GlaxoSmithKline (GSK) |
| Last Modified Date | 31 January 2026 |
Pneumococcal Vaccines Market Overview
Customize Pneumococcal Vaccines Market Report market research report
- ✔ Get in-depth analysis of Pneumococcal Vaccines market size, growth, and forecasts.
- ✔ Understand Pneumococcal Vaccines's regional dynamics and industry-specific trends.
- ✔ Identify potential applications, end-user demand, and growth segments in Pneumococcal Vaccines
What is the Market Size & CAGR of Pneumococcal Vaccines market in 2023?
Pneumococcal Vaccines Industry Analysis
Pneumococcal Vaccines Market Segmentation and Scope
Tell us your focus area and get a customized research report.
Pneumococcal Vaccines Market Analysis Report by Region
Europe Pneumococcal Vaccines Market Report:
In Europe, the market is anticipated to expand from $1.78 billion in 2023 to $3.33 billion by 2033, facilitated by strong healthcare policies and widespread vaccination campaigns.Asia Pacific Pneumococcal Vaccines Market Report:
In the Asia Pacific region, the Pneumococcal Vaccines market is expected to grow from $1.05 billion in 2023 to $1.97 billion by 2033, driven by increasing healthcare expenditure and rising awareness about vaccination amongst populations.North America Pneumococcal Vaccines Market Report:
North America will see substantial growth, increasing from $2.12 billion to $3.97 billion from 2023 to 2033, largely due to robust healthcare infrastructure and high awareness regarding the benefits of vaccination.South America Pneumococcal Vaccines Market Report:
The South American market is projected to grow from $0.28 billion to $0.52 billion during the same period, supported by enhanced immunization programs and government initiatives to curb pneumococcal diseases.Middle East & Africa Pneumococcal Vaccines Market Report:
The Middle East and Africa market is expected to grow from $0.37 billion to $0.69 billion, driven by ongoing public health efforts and foreign investments in healthcare initiatives.Tell us your focus area and get a customized research report.
Pneumococcal Vaccines Market Analysis By Product
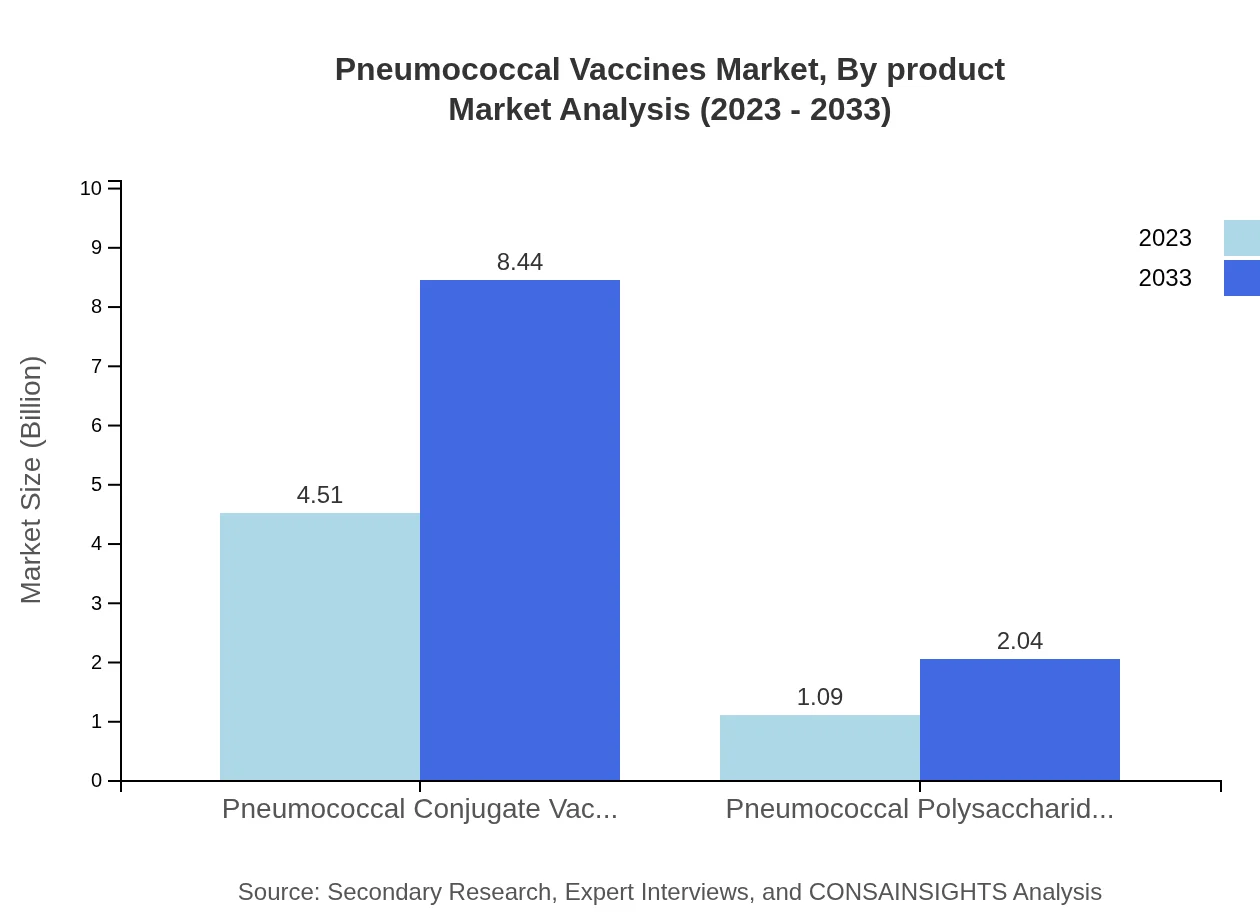
The Pneumococcal Vaccines Market, segmented by product, showcases significant growth in Pneumococcal Conjugate Vaccines (PCV) which account for a market size of $4.51 billion in 2023 and is forecasted to reach $8.44 billion by 2033, representing an 80.51% market share. Pneumococcal Polysaccharide Vaccines (PPV) are also notable, sized at $1.09 billion in 2023, expected to grow to $2.04 billion with a 19.49% market share.
Pneumococcal Vaccines Market Analysis By Age Group
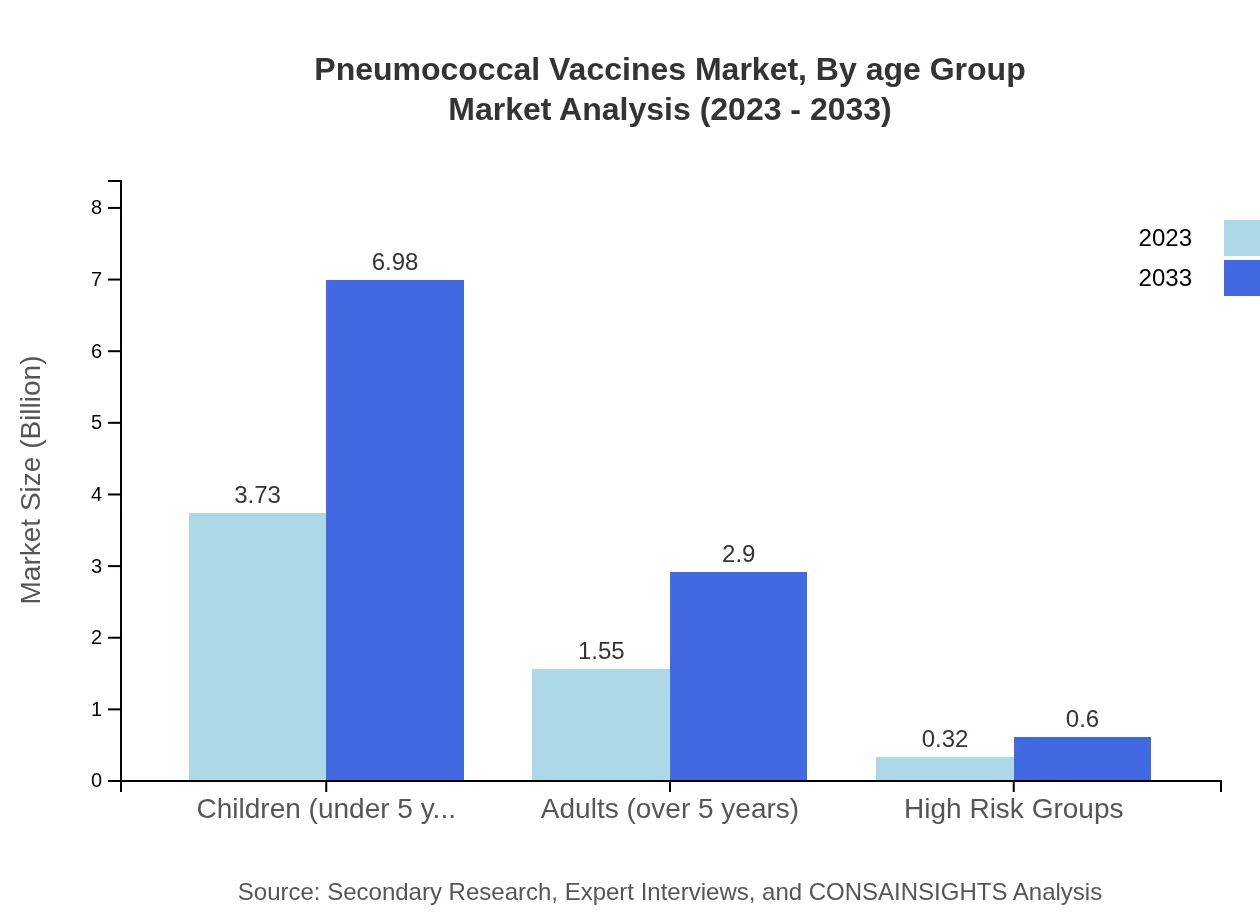
Segmenting by age group, the market for children under 5 years stands at $3.73 billion in 2023 with anticipated growth to $6.98 billion by 2033, maintaining a substantial share of around 66.63%. For adults over 5 years, the market is currently $1.55 billion and projected to rise to $2.90 billion, representing a 27.65% share, while high-risk groups encompass a smaller segment with a current size of $0.32 billion, expected to grow to $0.60 billion.
Pneumococcal Vaccines Market Analysis By Application
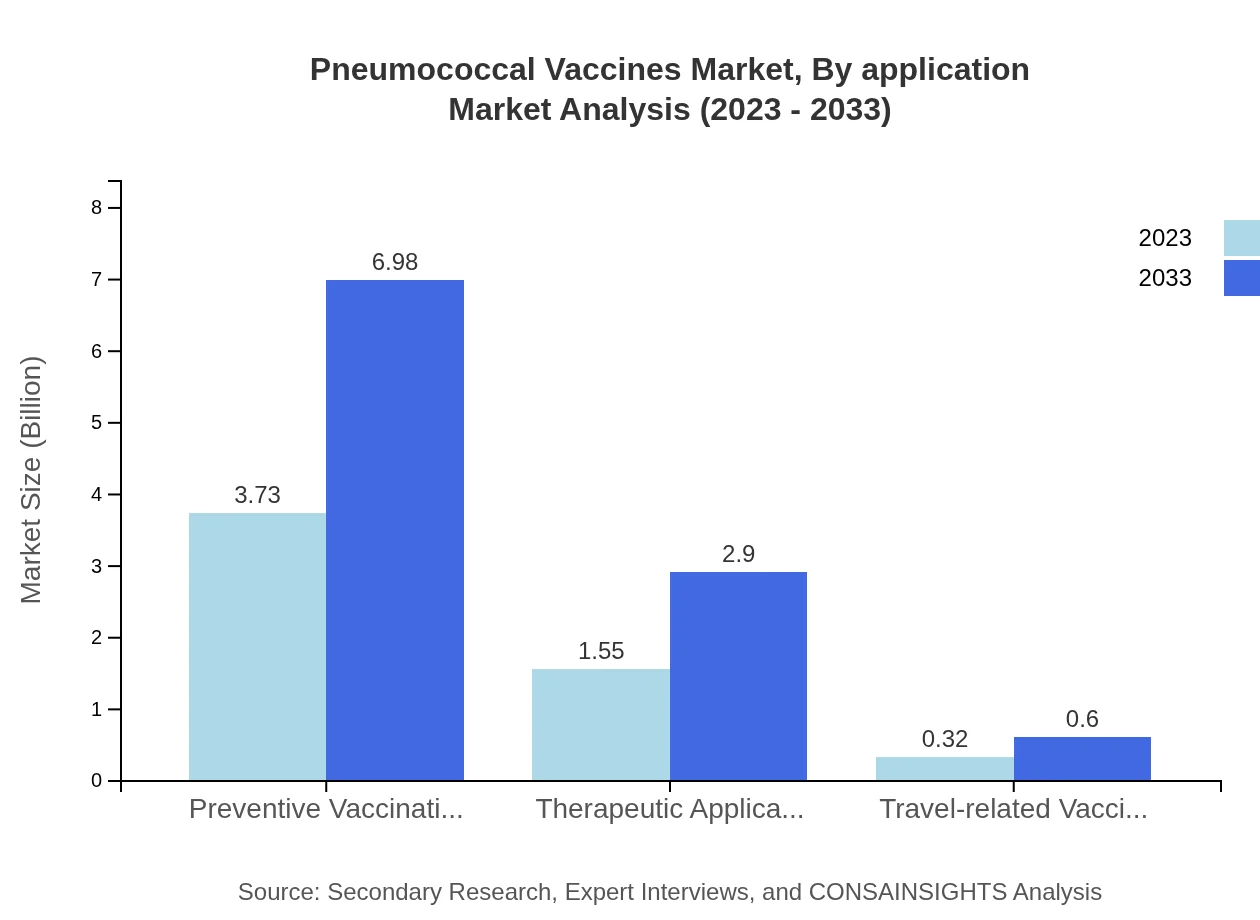
The application segment of preventive vaccinations accounts for a substantial market size of $3.73 billion in 2023, growing to $6.98 billion by 2033, constituting about 66.63% share. Therapeutic applications currently represent $1.55 billion, growing to $2.90 billion with a 27.65% share. Travel-related vaccination is comparatively smaller at $0.32 billion, expanding to $0.60 billion.
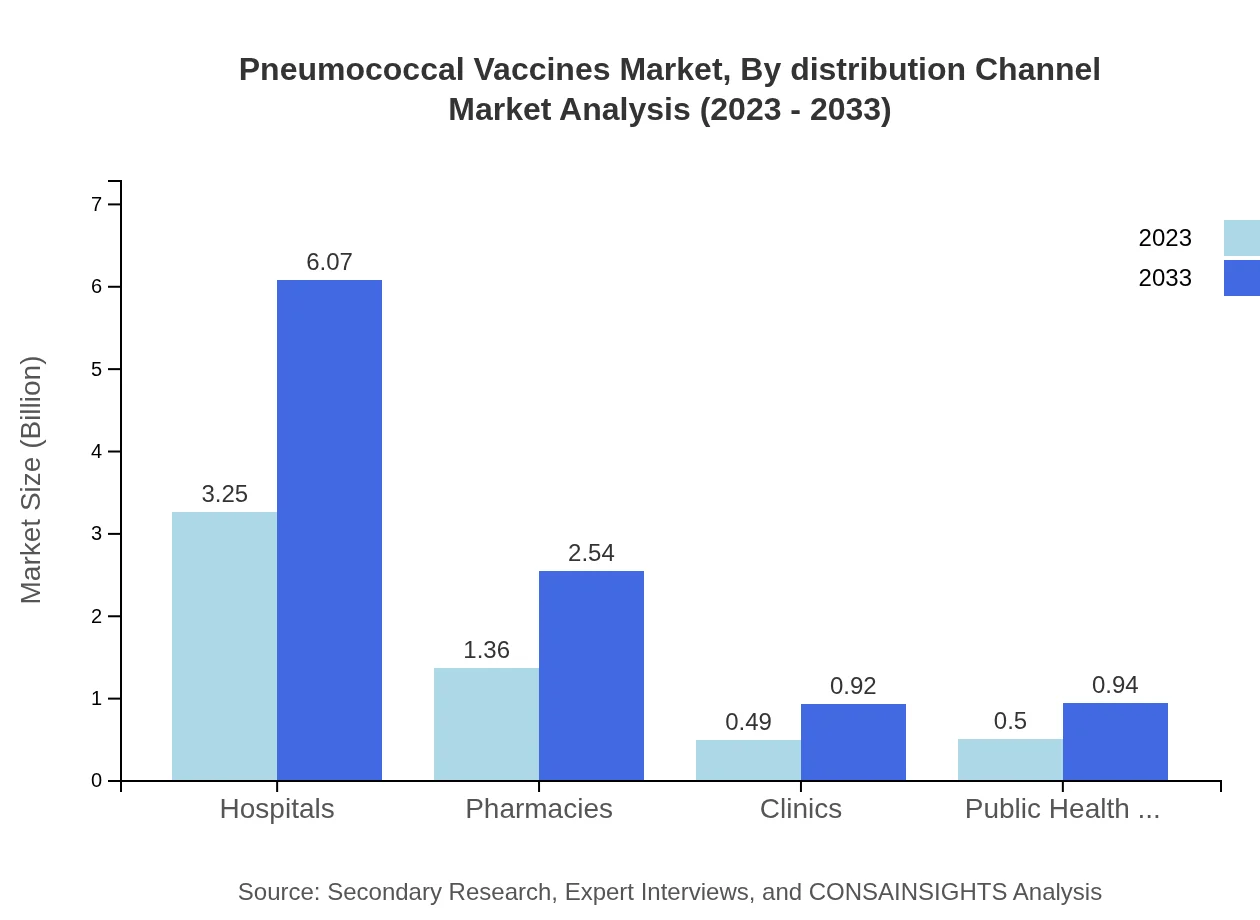
Pneumococcal Vaccines Market Analysis By Distribution Channel
Examining distribution channels, hospitals dominate the segment with a market size of $3.25 billion in 2023, anticipated to rise to $6.07 billion by 2033, holding a share of 57.97%. Pharmacies account for $1.36 billion, growing to $2.54 billion with 24.23%, while clinics represent a smaller $0.49 billion, projected to reach $0.92 billion in the coming years. Public health programs also play a crucial role with a market size of $0.50 billion expected to increase to $0.94 billion.
Pneumococcal Vaccines Market Trends and Future Forecast
Tell us your focus area and get a customized research report.
Global Market Leaders and Top Companies in Pneumococcal Vaccines Industry
Pfizer Inc.:
A leading pharmaceutical company with a strong presence in the immunization sector, known for its pneumococcal conjugate vaccine Prevnar 13.Merck & Co., Inc.:
A global healthcare company recognized for its contributions to vaccine development, particularly the Pneumococcal polysaccharide vaccine Pneumovax 23.Sanofi Pasteur:
The vaccines division of Sanofi, contributing innovative pneumococcal vaccines and actively involved in global vaccination initiatives.GlaxoSmithKline (GSK):
A research-based company known for its vaccines, GSK is pivotal in providing pneumococcal vaccines and expanding immunization reach.We're grateful to work with incredible clients.









FAQs
What is the market size of pneumococcal vaccines?
The global pneumococcal vaccines market was valued at $5.6 billion in 2023 and is projected to grow at a CAGR of 6.3% through 2033. Such growth reflects rising awareness and health initiatives aimed at combatting pneumococcal diseases.
What are the key market players or companies in the pneumococcal vaccines industry?
Key players include major pharmaceutical companies such as Pfizer, Sanofi, Merck, and GlaxoSmithKline. These companies lead in vaccine development, distribution, and innovative research contributing to market expansion.
What are the primary factors driving the growth in the pneumococcal vaccines industry?
Factors include increased immunization rates, awareness regarding pneumococcal disease prevention, rising elderly population, and government policies promoting vaccination programs, which together enhance demand for pneumococcal vaccines.
Which region is the fastest Growing in the pneumococcal vaccines market?
The fastest-growing region is Europe, projected to grow from $1.78 billion in 2023 to $3.33 billion by 2033. Strong public health initiatives and increased vaccine accessibility support this growth trajectory.
Does ConsaInsights provide customized market report data for the pneumococcal vaccines industry?
Yes, ConsaInsights offers tailored market report data that can be customized to meet specific client needs, focusing on unique market segments or regional insights within the pneumococcal vaccines industry.
What deliverables can I expect from this pneumococcal vaccines market research project?
Clients can expect comprehensive market analyses, including market size estimates, growth forecasts, competitive landscape evaluations, and region-specific insights, tailored to the client's strategic needs.
What are the market trends of pneumococcal vaccines?
Current trends include rising demand for pneumococcal conjugate vaccines (PCV), growing vaccination of high-risk groups, and expansion of vaccination programs in low and middle-income countries, impacting overall market dynamics.