Pneumonia Testing Market Report
Published Date: 31 January 2026 | Report Code: pneumonia-testing
Pneumonia Testing Market Size, Share, Industry Trends and Forecast to 2033
This report provides a comprehensive analysis of the Pneumonia Testing market, including market insights, growth forecasts, and segmentation details for the period 2023-2033. It highlights current market conditions, technological advancements, and regional variances.
| Metric | Value |
|---|---|
| Study Period | 2023 - 2033 |
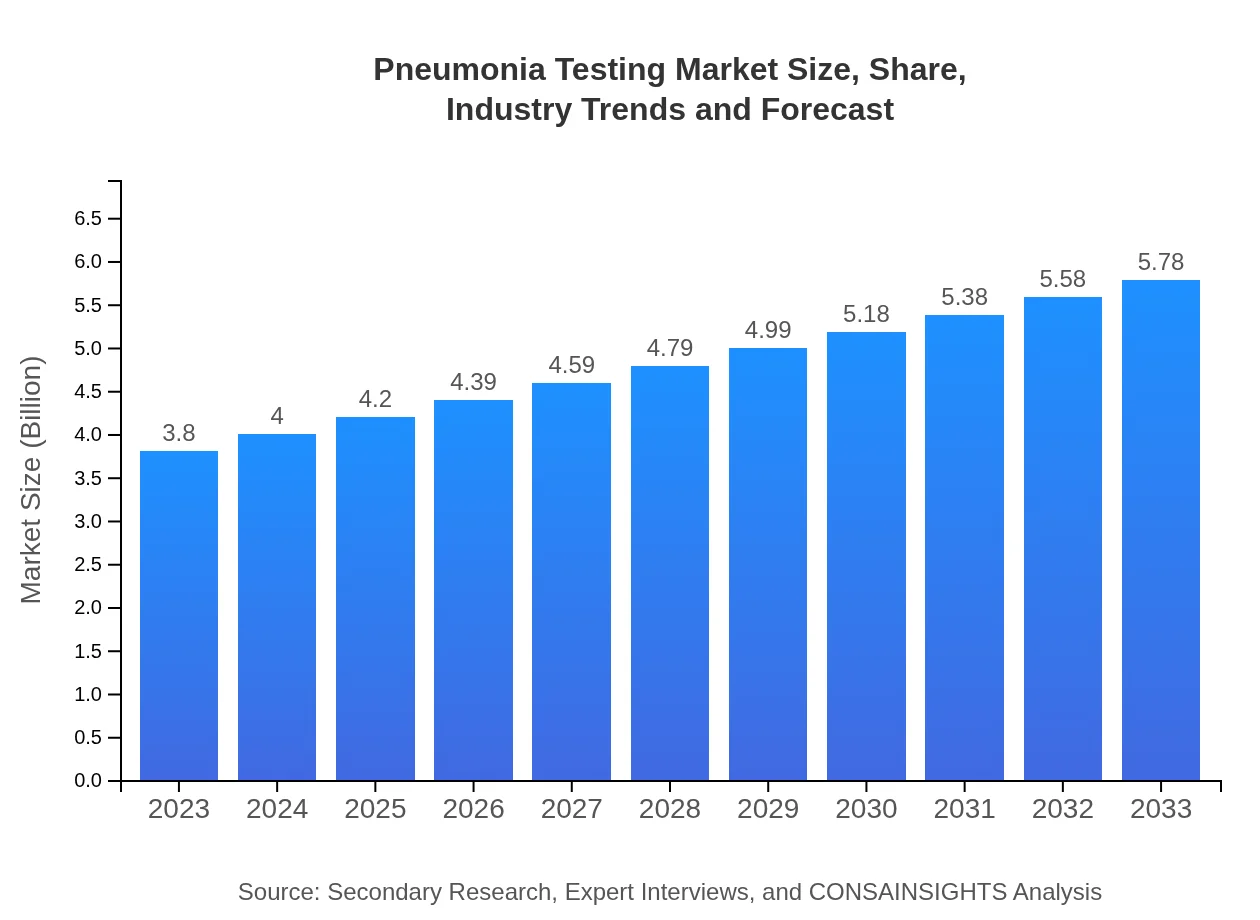
| 2023 Market Size | $3.80 Billion |
| CAGR (2023-2033) | 4.2% |
| 2033 Market Size | $5.78 Billion |
| Top Companies | Roche Diagnostics, Abbott Laboratories, Thermo Fisher Scientific, Quidel Corporation |
| Last Modified Date | 31 January 2026 |
Pneumonia Testing Market Overview
Customize Pneumonia Testing Market Report market research report
- ✔ Get in-depth analysis of Pneumonia Testing market size, growth, and forecasts.
- ✔ Understand Pneumonia Testing's regional dynamics and industry-specific trends.
- ✔ Identify potential applications, end-user demand, and growth segments in Pneumonia Testing
What is the Market Size & CAGR of Pneumonia Testing market in 2023?
Pneumonia Testing Industry Analysis
Pneumonia Testing Market Segmentation and Scope
Tell us your focus area and get a customized research report.
Pneumonia Testing Market Analysis Report by Region
Europe Pneumonia Testing Market Report:
Europe's Pneumonia Testing market is anticipated to grow from 1.07 billion USD in 2023 to 1.63 billion USD by 2033, propelled by stringent health regulations and increasing awareness of respiratory diseases.Asia Pacific Pneumonia Testing Market Report:
The Asia Pacific region is projected to see significant market growth, with the market expected to increase from 0.72 billion USD in 2023 to 1.09 billion USD by 2033. Factors include rising healthcare expenditure and growing awareness of pneumonia.North America Pneumonia Testing Market Report:
North America holds the largest share of the Pneumonia Testing market, expected to increase from 1.45 billion USD in 2023 to 2.20 billion USD in 2033. This growth is driven by high healthcare spending and advanced technological adoption.South America Pneumonia Testing Market Report:
In South America, the Pneumonia Testing market is estimated to grow from 0.10 billion USD in 2023 to 0.15 billion USD by 2033. Increased focus on healthcare infrastructure and public health initiatives is driving growth.Middle East & Africa Pneumonia Testing Market Report:
This region is expected to experience growth from 0.46 billion USD in 2023 to 0.70 billion USD by 2033, influenced by improving healthcare systems and rising disease awareness.Tell us your focus area and get a customized research report.
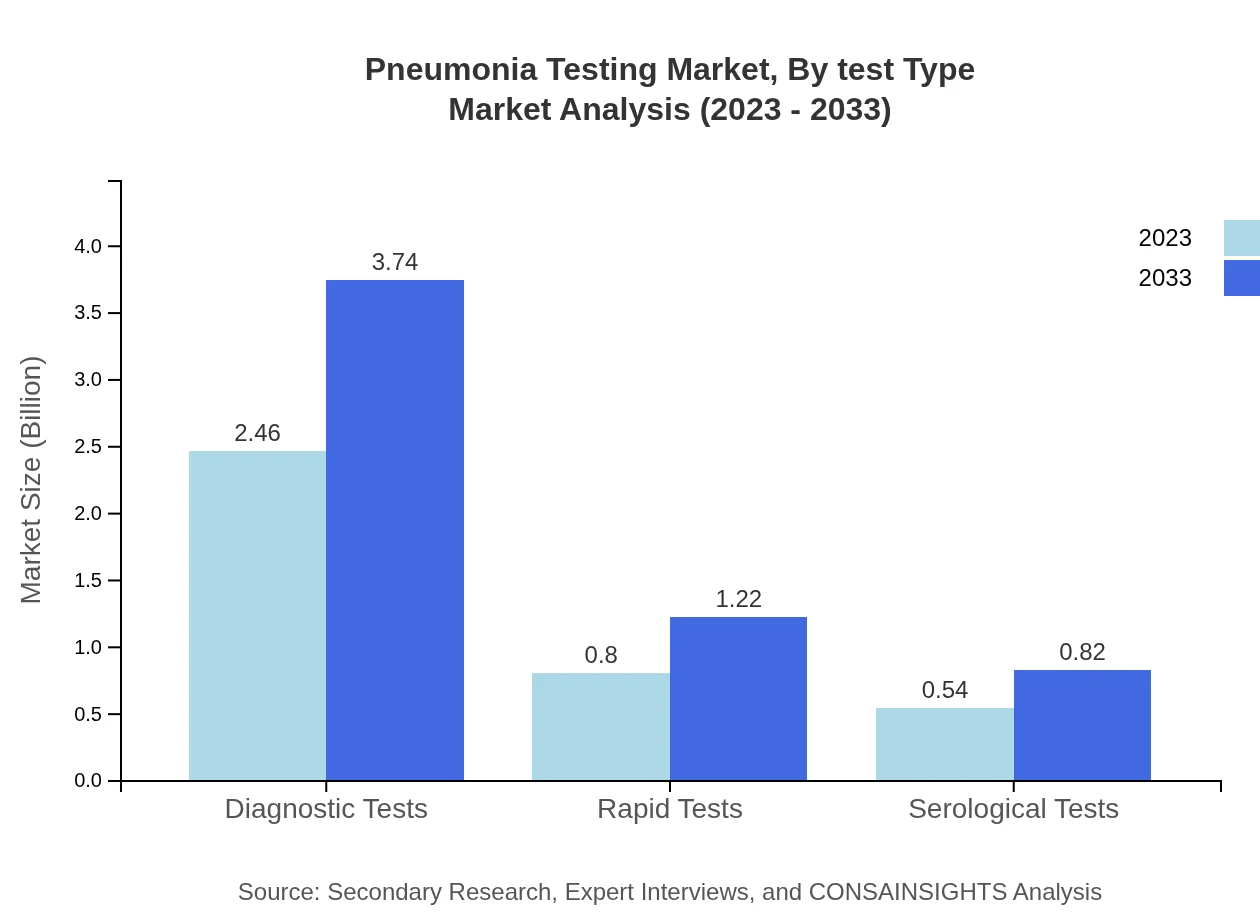
Pneumonia Testing Market Analysis By Test Type
The market by test type shows that Diagnostic Tests dominate with a market share of 64.72% in 2023, projected to grow to 64.72% by 2033. Molecular diagnostics also hold significant value, showing continued investment in this area. Rapid tests and immunodiagnostics are emerging trends within this segment, indicating a shift towards quicker and more efficient testing methods.
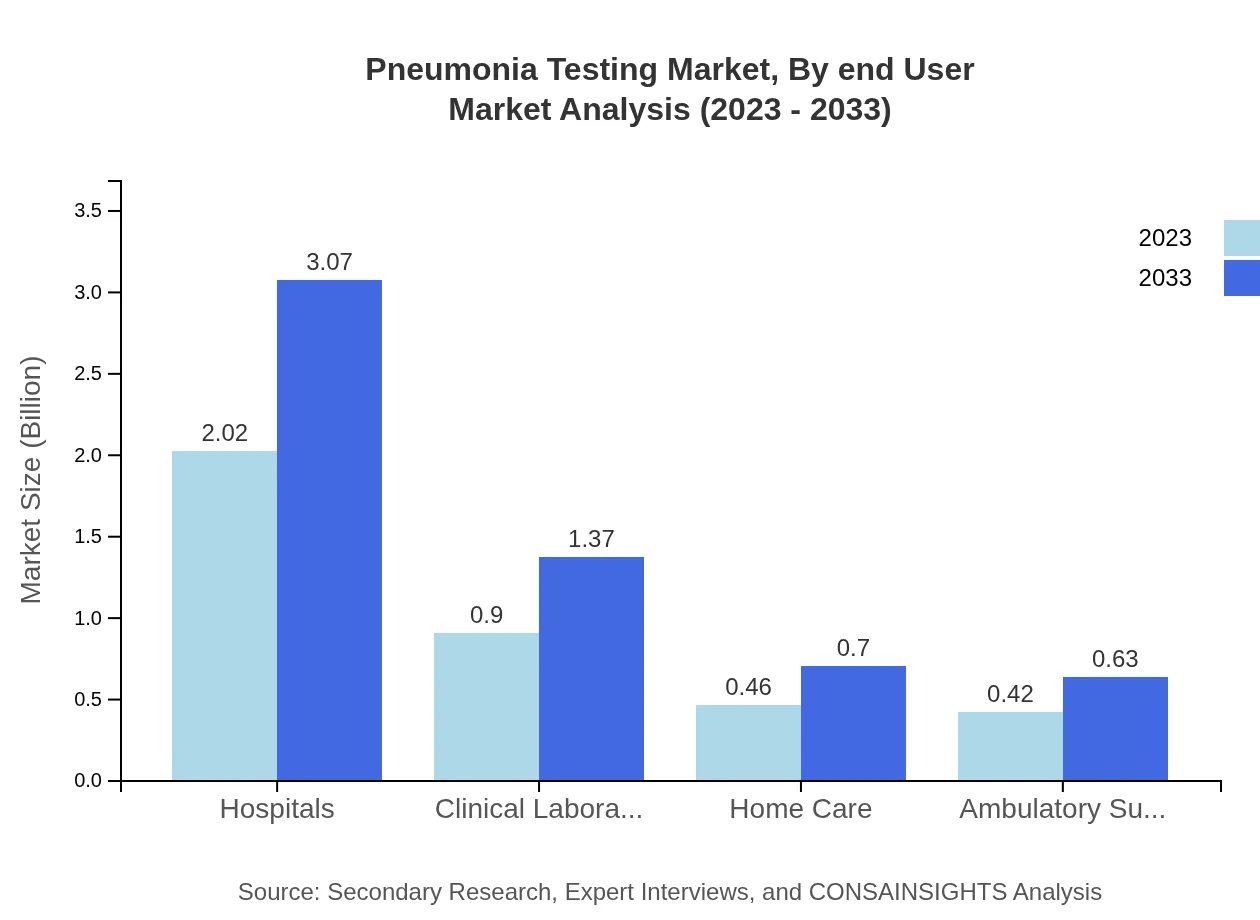
Pneumonia Testing Market Analysis By End User
Hospitals are the leading end-user segment with a market size of 2.02 billion USD in 2023, expected to reach 3.07 billion USD in 2033. Clinical laboratories and home care also present significant market sizes, indicating a diversification in end-user settings for pneumonia testing.
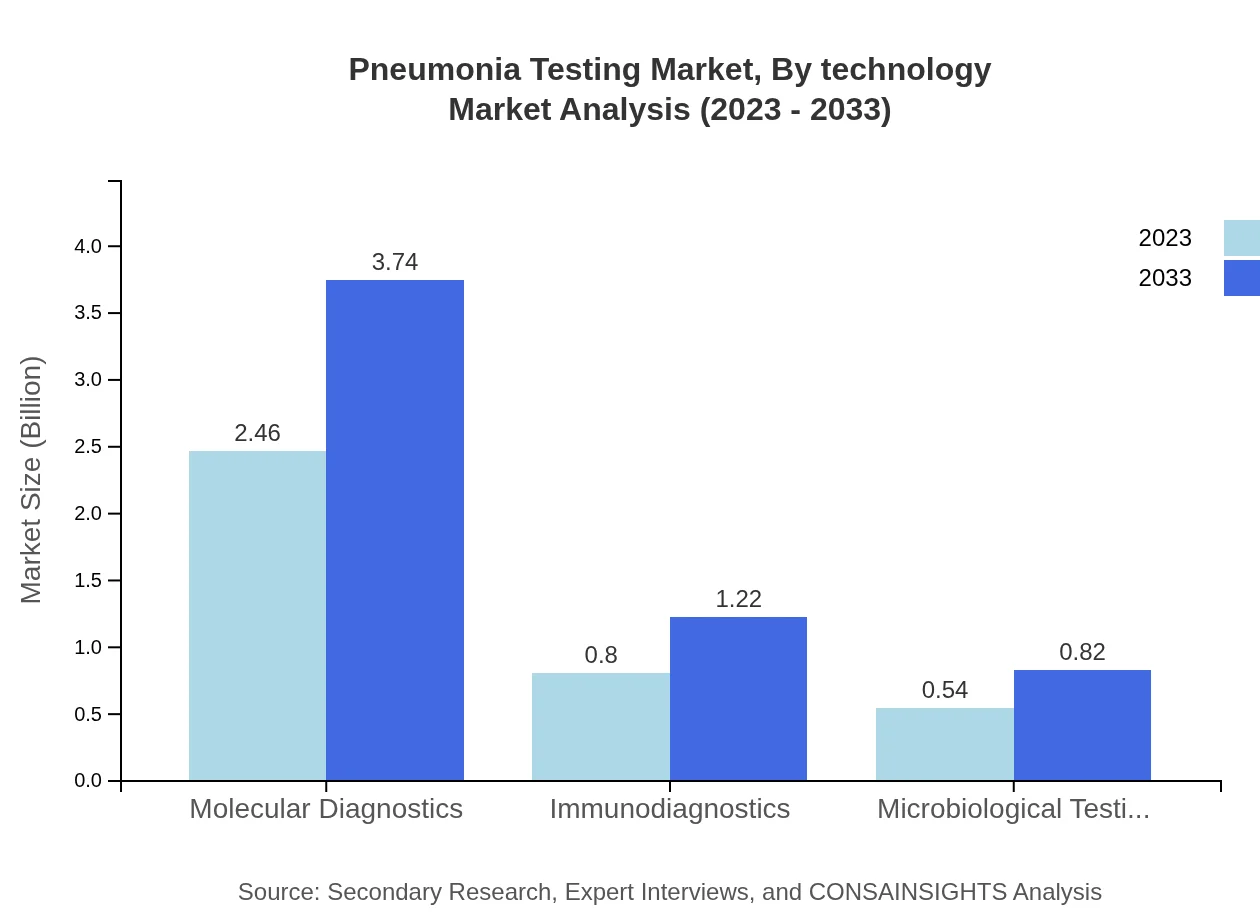
Pneumonia Testing Market Analysis By Technology
The technological segment showcases Diagnostic Tests' leading position, accounting for significant market size in 2023 with projected growth. Rapid tests are gaining traction due to their quick results, alongside increasing preference for immunodiagnostics in clinical settings.
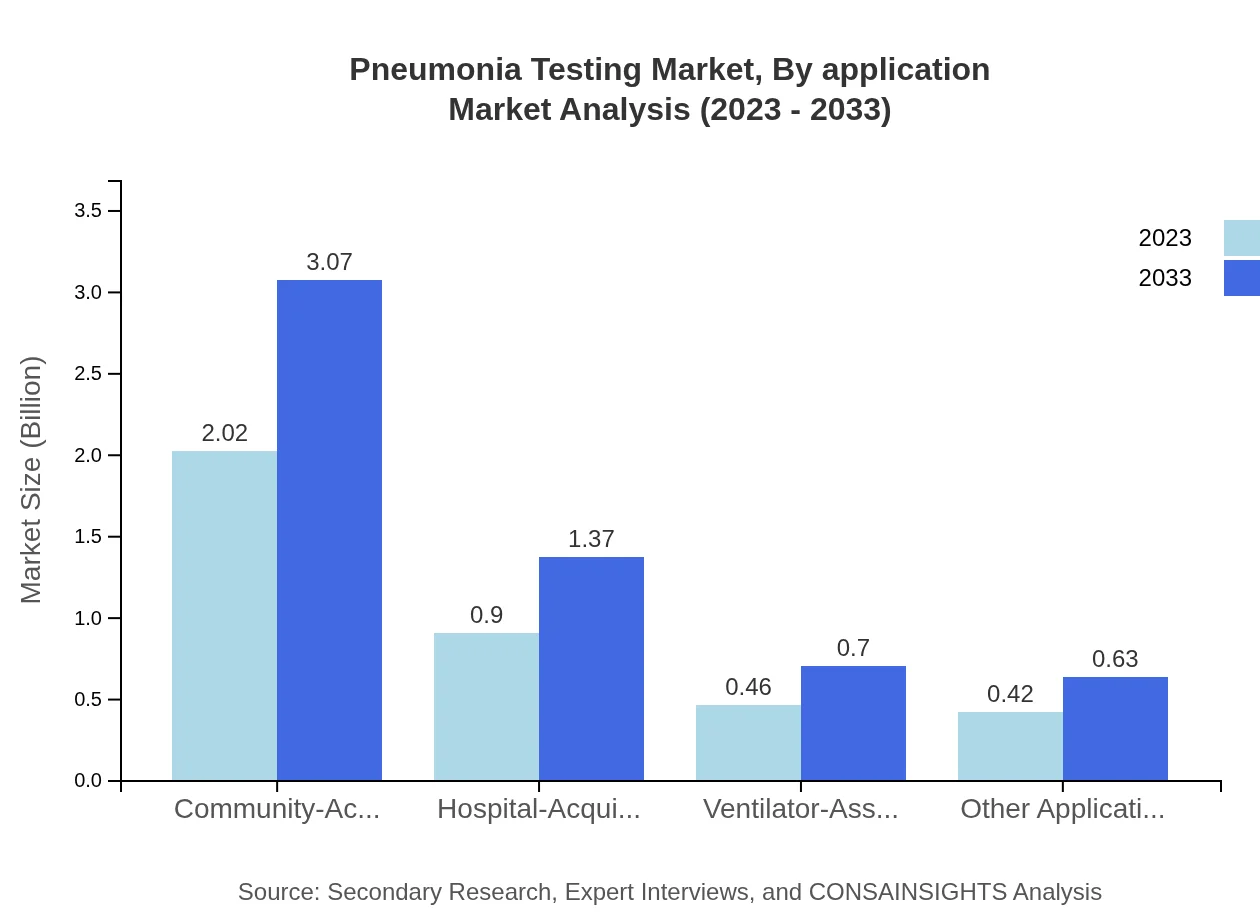
Pneumonia Testing Market Analysis By Application
Community-acquired pneumonia testing represents the largest application area, holding a significant share of 53.2% in 2023. This segment is expected to retain its importance through 2033, while other applications like hospital-acquired and ventilator-associated pneumonia are also growing steadily.
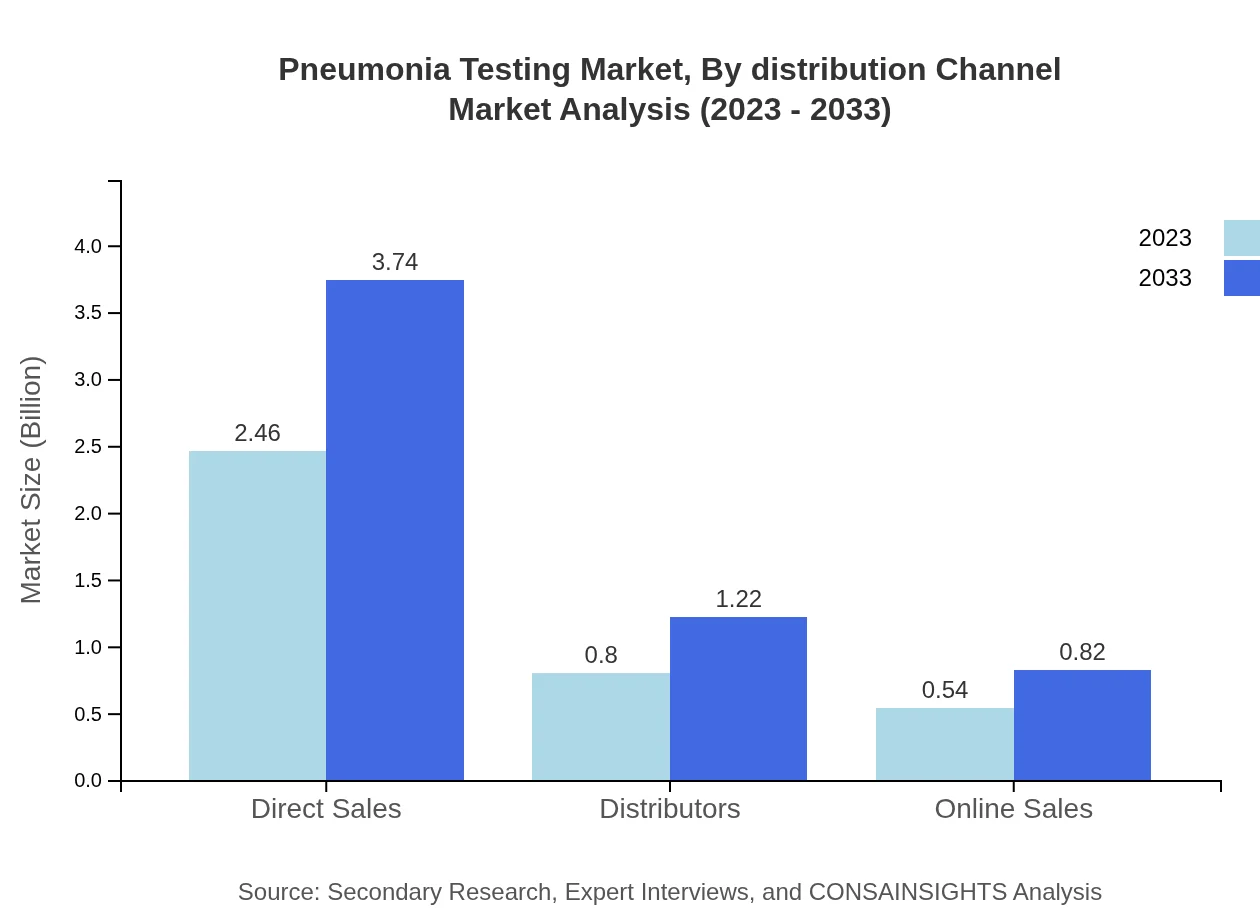
Pneumonia Testing Market Analysis By Distribution Channel
Direct sales dominate the distribution channels for pneumonia testing, maintaining a share of 64.72% in 2023, projected to continue through 2033. Online sales are also expected to grow, reflecting changing consumer behaviors in health-related purchases.
Pneumonia Testing Market Trends and Future Forecast
Tell us your focus area and get a customized research report.
Global Market Leaders and Top Companies in Pneumonia Testing Industry
Roche Diagnostics:
A leader in innovative diagnostic solutions, Roche Diagnostics is committed to advancing pneumonia testing through cutting-edge technology and research.Abbott Laboratories:
Known for its comprehensive diagnostic equipment, Abbott is dedicated to improving healthcare outcomes through rapid testing innovations for pneumonia.Thermo Fisher Scientific:
Thermo Fisher offers a wide range of diagnostic solutions, playing a crucial role in the molecular diagnostics segment for pneumonia testing.Quidel Corporation:
Quidel specializes in rapid diagnostic testing, particularly for infectious diseases, contributing to prompt pneumonia diagnosis.We're grateful to work with incredible clients.









FAQs
What is the market size of pneumonia Testing?
The global pneumonia testing market is currently valued at approximately $3.8 billion, with a projected compound annual growth rate (CAGR) of 4.2% from 2023 to 2033, indicating steady growth driven by increasing healthcare awareness and advancements in diagnostic technologies.
What are the key market players or companies in this pneumonia Testing industry?
Key players in the pneumonia testing market include leading healthcare companies and diagnostic laboratories that specialize in infectious disease diagnostics. These companies are leveraging innovative technologies such as molecular diagnostics and rapid testing to cater to the rising demand for effective pneumonia testing.
What are the primary factors driving the growth in the pneumonia Testing industry?
The pneumonia testing industry is primarily driven by factors such as the growing incidence of respiratory infections, advancements in diagnostic technologies, increased healthcare expenditure, and heightened awareness about pneumonia among patients and healthcare providers, leading to early detection and management.
Which region is the fastest Growing in the pneumonia testing market?
Among global regions, the Asia Pacific is expected to witness the fastest growth in the pneumonia testing market, rising from $0.72 billion in 2023 to $1.09 billion by 2033, primarily due to increasing healthcare infrastructure and rising awareness of infectious diseases.
Does ConsaInsights provide customized market report data for the pneumonia testing industry?
Yes, ConsaInsights offers tailored market reports for the pneumonia testing industry, allowing clients to access specific data and insights tailored to their need, including segmented analysis as well as regional breakdowns to support decision-making.
What deliverables can I expect from this pneumonia testing market research project?
From the pneumonia testing market research project, clients can expect comprehensive reports featuring market size data, growth forecasts, competitive landscape analysis, trend identification, and regional breakdowns to support strategic planning and investment decisions.
What are the market trends of pneumonia Testing?
Current trends in the pneumonia testing market include the increased adoption of rapid testing methods, enhanced focus on molecular diagnostics, and the integration of technology in healthcare, all of which are shaping the future landscape of pneumonia diagnostics.