Point Of Care Infectious Disease Market Report
Published Date: 31 January 2026 | Report Code: point-of-care-infectious-disease
Point Of Care Infectious Disease Market Size, Share, Industry Trends and Forecast to 2033
This report provides an extensive analysis of the Point Of Care Infectious Disease market, covering insights into market size, trends, and forecasts from 2023 to 2033, with a focus on regional performance and technological advancements.
| Metric | Value |
|---|---|
| Study Period | 2023 - 2033 |
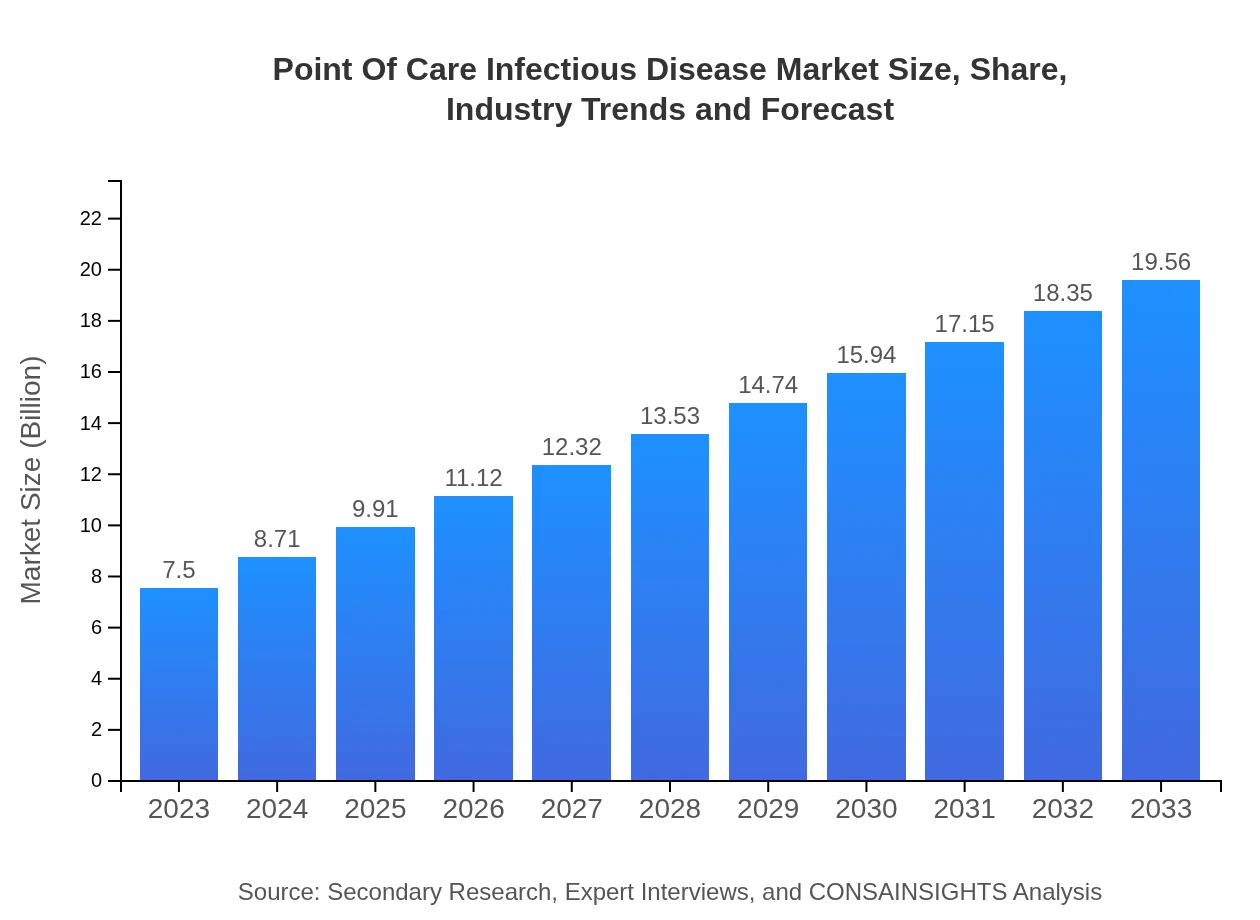
| 2023 Market Size | $7.50 Billion |
| CAGR (2023-2033) | 9.7% |
| 2033 Market Size | $19.56 Billion |
| Top Companies | Roche Diagnostics, Abbott Laboratories, Siemens Healthineers |
| Last Modified Date | 31 January 2026 |
Point Of Care Infectious Disease Market Overview
Customize Point Of Care Infectious Disease Market Report market research report
- ✔ Get in-depth analysis of Point Of Care Infectious Disease market size, growth, and forecasts.
- ✔ Understand Point Of Care Infectious Disease's regional dynamics and industry-specific trends.
- ✔ Identify potential applications, end-user demand, and growth segments in Point Of Care Infectious Disease
What is the Market Size & CAGR of Point Of Care Infectious Disease market in 2023 and 2033?
Point Of Care Infectious Disease Industry Analysis
Point Of Care Infectious Disease Market Segmentation and Scope
Tell us your focus area and get a customized research report.
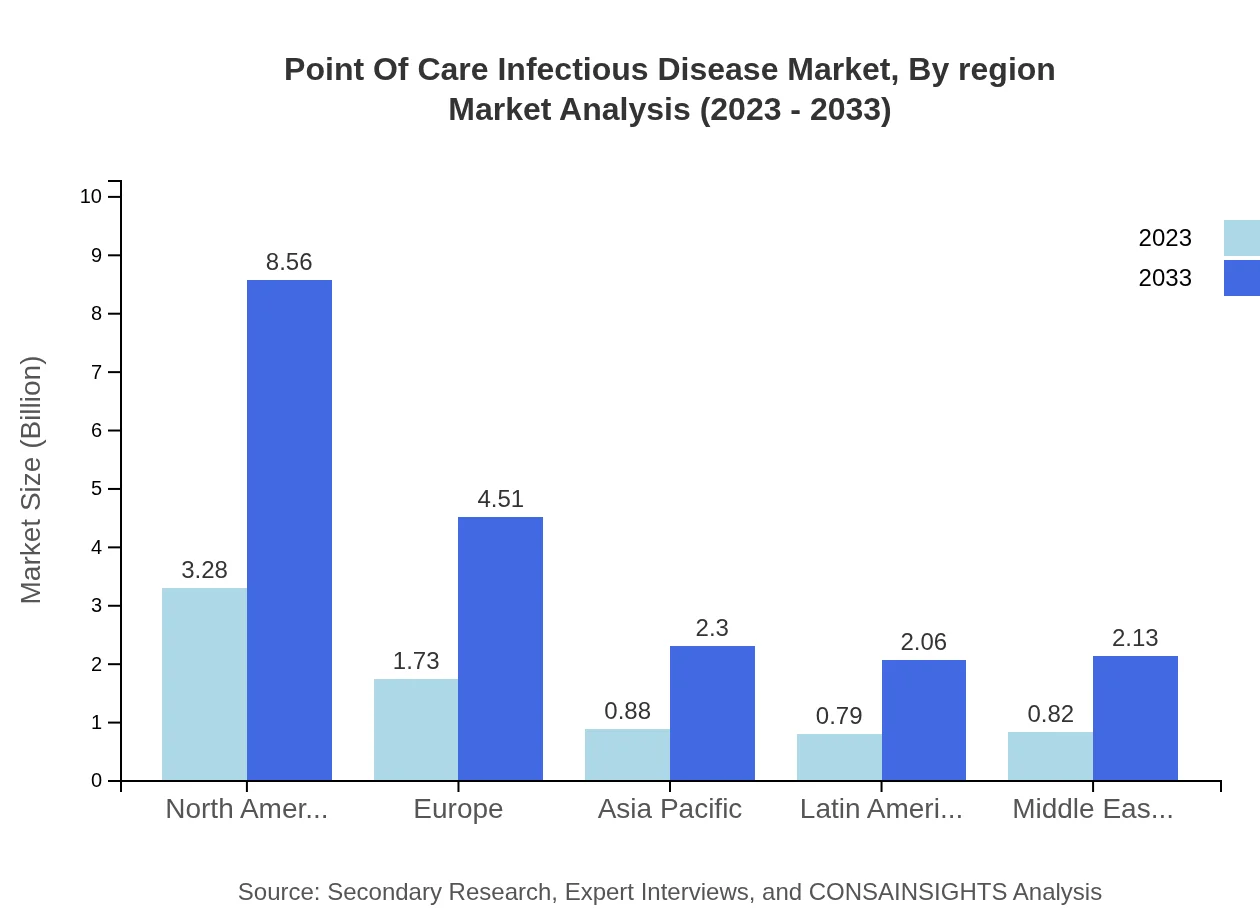
Point Of Care Infectious Disease Market Analysis Report by Region
Europe Point Of Care Infectious Disease Market Report:
Europe is projected to show significant growth in the POC infectious disease market, rising from $2.27 billion in 2023 to $5.91 billion by 2033. The region's focus on decentralized healthcare and the adoption of innovative technologies will support this growth.Asia Pacific Point Of Care Infectious Disease Market Report:
The Asia Pacific region is expected to experience substantial growth, reflecting an increase from $1.53 billion in 2023 to $3.99 billion by 2033. The rising prevalence of infectious diseases, along with rapid urbanization and improving healthcare infrastructure, are key drivers.North America Point Of Care Infectious Disease Market Report:
In North America, the market size will expand from $2.56 billion in 2023 to $6.67 billion by 2033, fueled by advanced healthcare facilities, increasing investments in POC technologies, and a higher incidence of infectious diseases.South America Point Of Care Infectious Disease Market Report:
The South American market is anticipated to grow from $0.46 billion in 2023 to $1.19 billion by 2033. The increased focus on improving healthcare access and the need for rapid testing solutions are prominent growth factors.Middle East & Africa Point Of Care Infectious Disease Market Report:
The Middle East and Africa region is expected to grow from $0.69 billion in 2023 to $1.80 billion by 2033, driven by increasing healthcare expenditure and the urgent need for effective disease management.Tell us your focus area and get a customized research report.
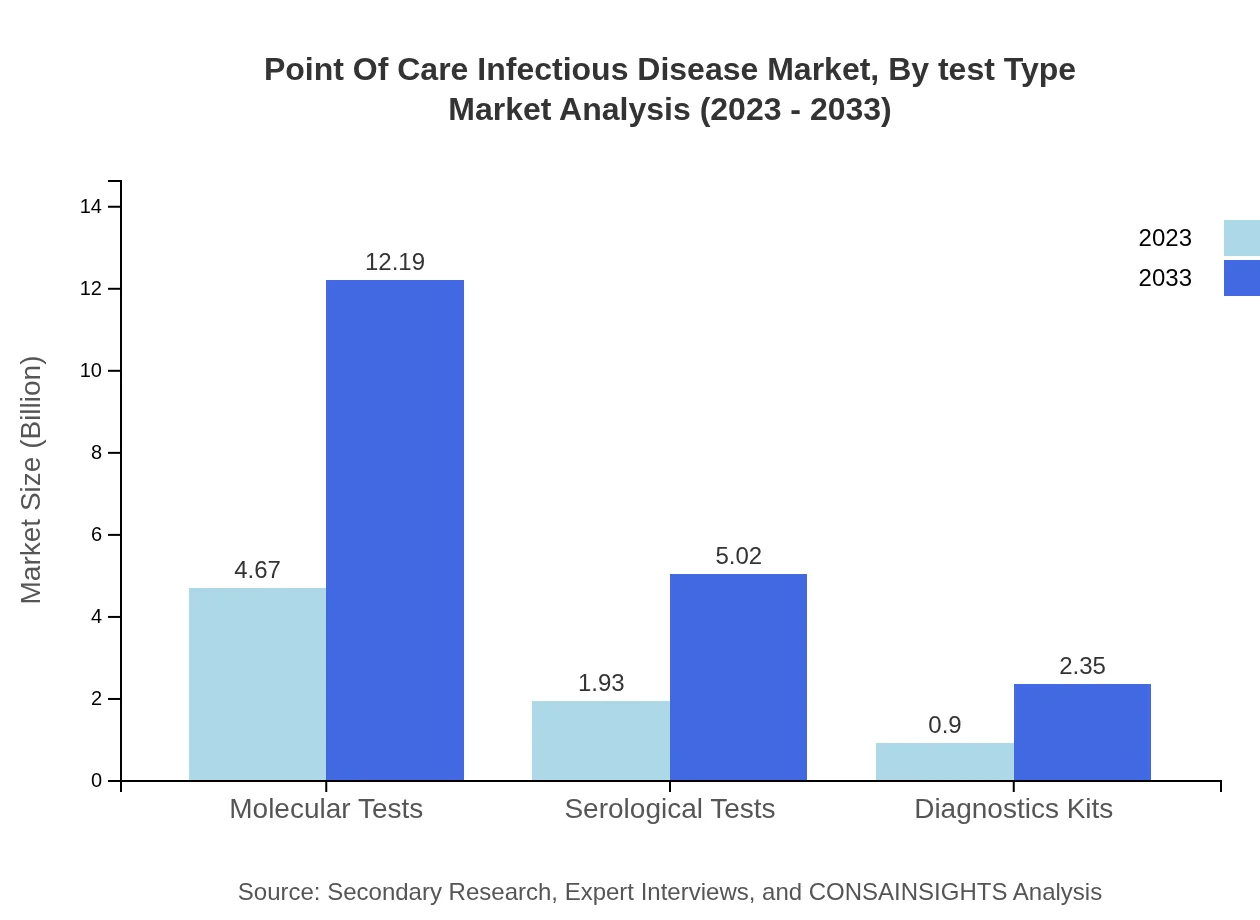
Point Of Care Infectious Disease Market Analysis By Test Type
The market segmentation by test type shows significant differentiation in performance. Molecular tests, being the most accurate, accounted for $4.67 billion in 2023 and are expected to rise to $12.19 billion by 2033, holding a share of 62.32%. Meanwhile, serological tests are projected to grow from $1.93 billion to $5.02 billion, reflecting their essential role in disease diagnosis.
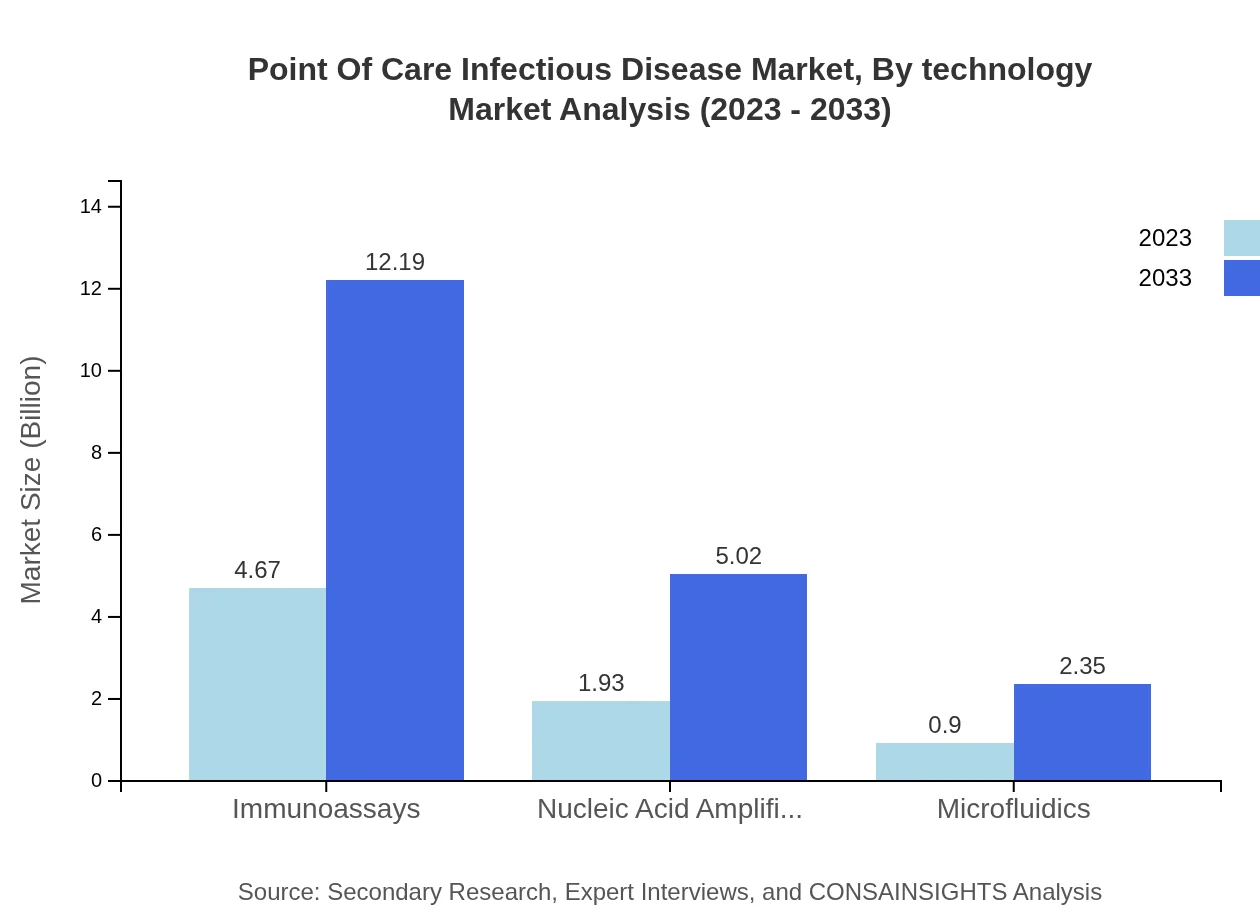
Point Of Care Infectious Disease Market Analysis By Technology
The technology segment reflects diverse innovations, with immunoassays leading the market with a size of $4.67 billion in 2023, projected to reach $12.19 billion by 2033, constituting 62.32% of the market share. Other technologies such as nucleic acid amplification are also significant contributors.
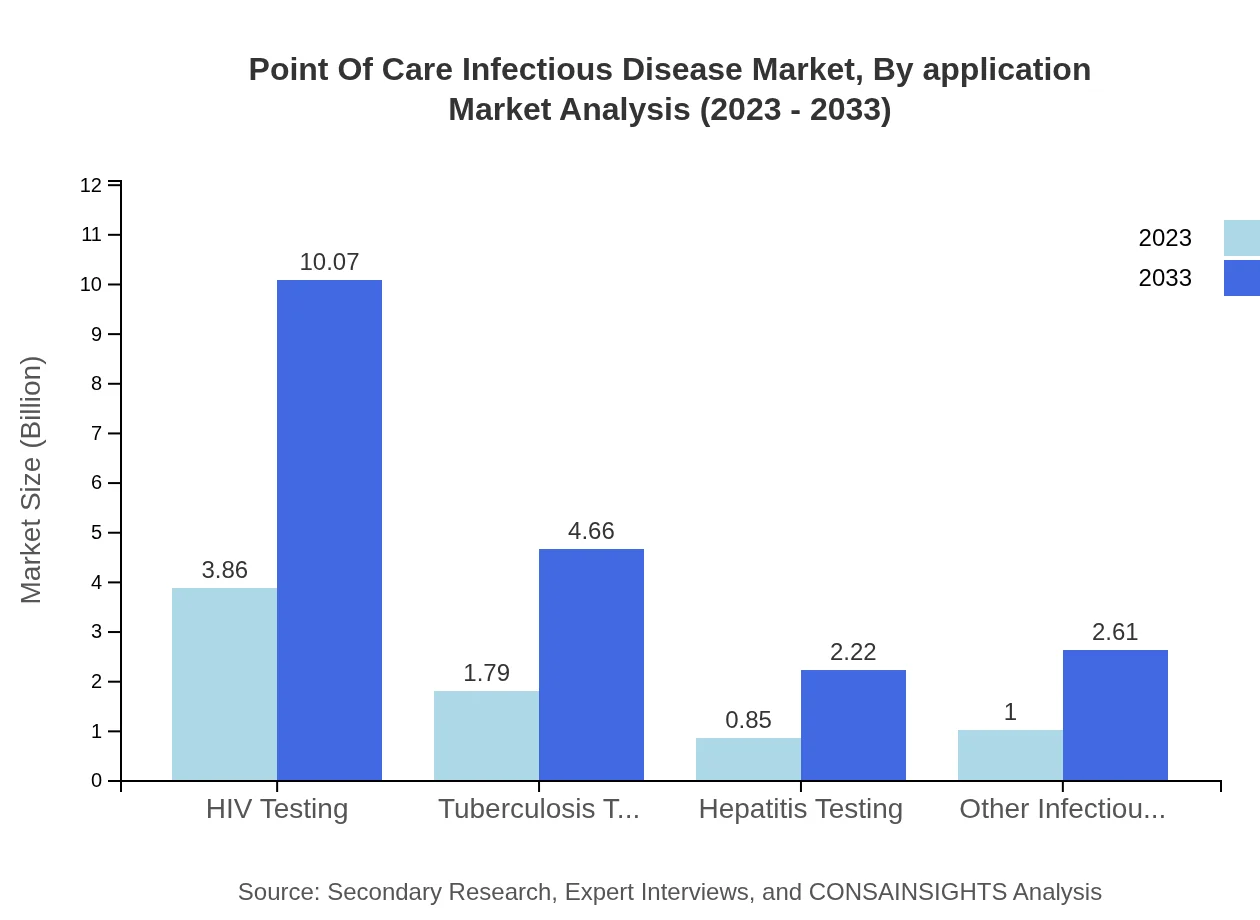
Point Of Care Infectious Disease Market Analysis By Application
Applications range across various infectious diseases, and HIV testing shows the largest market size, expected to grow from $3.86 billion in 2023 to $10.07 billion by 2033. Tuberculosis testing and hepatitis testing also contribute significantly to the market landscape.
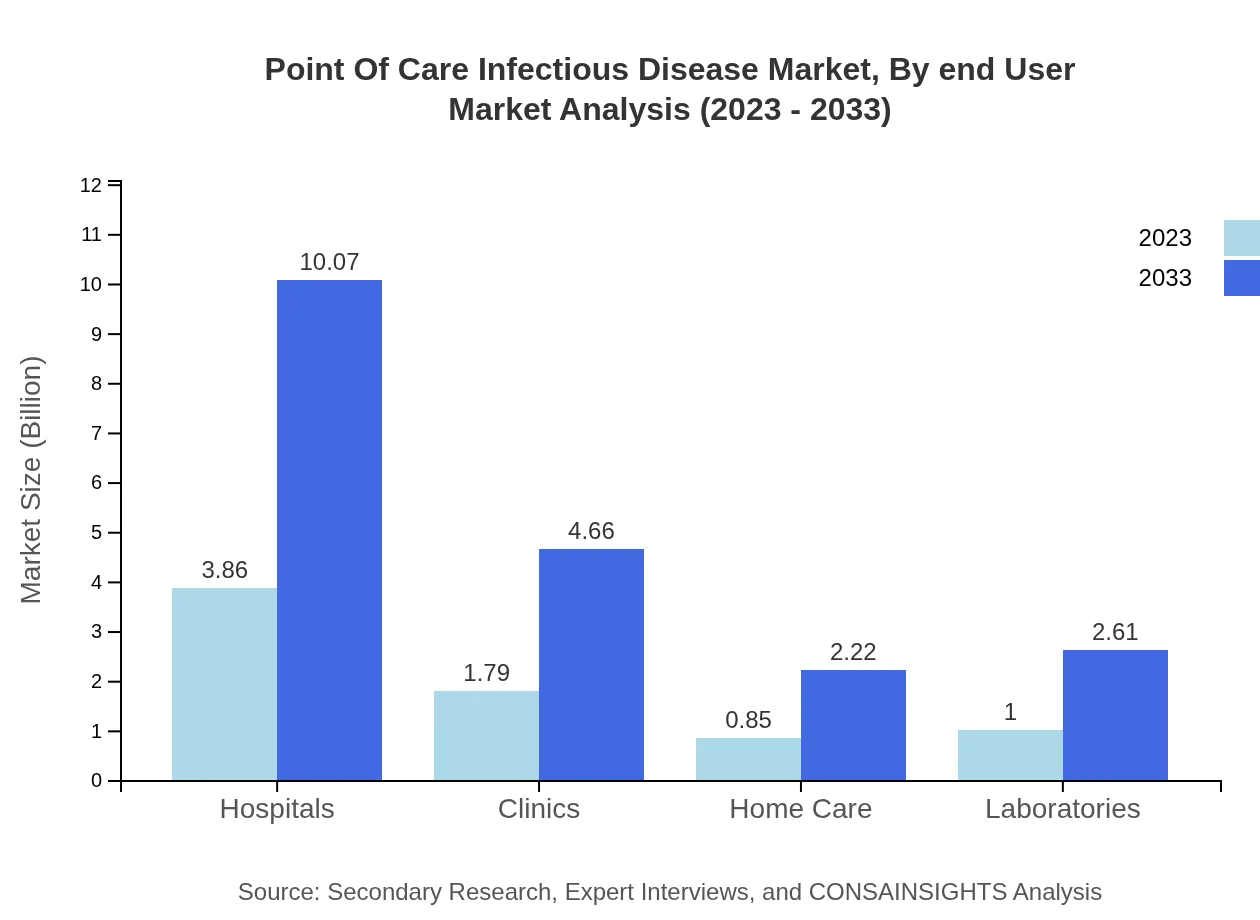
Point Of Care Infectious Disease Market Analysis By End User
Hospitals emerge as the primary end-user segment, with growth anticipated from $3.86 billion to $10.07 billion. Clinics and home care are also gaining traction, reflecting a shift towards decentralized diagnostic approaches.
Point Of Care Infectious Disease Market Analysis By Region
The regional analysis identifies North America, Europe, and Asia-Pacific as leading markets, with substantial contributions to overall growth. Each region's unique needs drive demand for specific diagnostic solutions.
Point Of Care Infectious Disease Market Trends and Future Forecast
Tell us your focus area and get a customized research report.
Global Market Leaders and Top Companies in Point Of Care Infectious Disease Industry
Roche Diagnostics:
A global leader in in-vitro diagnostics, Roche offers a wide range of POC infectious disease testing solutions, integrating innovative technologies.Abbott Laboratories:
Abbott is renowned for its cutting-edge diagnostic tools and has significantly contributed to the POC market, especially in molecular diagnostics.Siemens Healthineers:
Specializing in medical technology, Siemens Healthineers provides advanced POC testing instruments and solutions focusing on infectious diseases.We're grateful to work with incredible clients.









FAQs
What is the market size of point Of Care Infectious Disease?
The global point-of-care infectious disease market is valued at approximately $7.5 billion in 2023, with a projected compound annual growth rate (CAGR) of 9.7%, anticipating significant growth over the next decade.
What are the key market players or companies in this point Of Care Infectious Disease industry?
Key players in the point-of-care infectious disease market include Abbott Laboratories, Cepheid, Quidel Corporation, Hologic, and Roche Diagnostics, along with emerging companies focusing on innovative diagnostic technologies.
What are the primary factors driving the growth in the point Of Care Infectious Disease industry?
Factors driving growth include increasing prevalence of infectious diseases, rising demand for rapid diagnostic tests, advancements in technology, and a shift towards decentralized healthcare, enabling quicker patient management.
Which region is the fastest Growing in the point Of Care Infectious Disease?
Asia-Pacific is the fastest-growing region in the point-of-care infectious disease market, with market growth projected from $1.53 billion in 2023 to $3.99 billion by 2033, reflecting robust demand.
Does ConsaInsights provide customized market report data for the point Of Care Infectious Disease industry?
Yes, ConsaInsights offers customized market report data tailored to specific needs in the point-of-care infectious disease industry, providing insights that align with client requirements.
What deliverables can I expect from this point Of Care Infectious Disease market research project?
Deliverables typically include detailed market analysis reports, segmentation insights, competitive landscape evaluations, growth projections, and actionable recommendations based on thorough research.
What are the market trends of point Of Care Infectious Disease?
Emerging trends include the adoption of molecular diagnostics, increasing integration of telemedicine, rising awareness of rapid testing solutions, and enhanced investment in research for better infectious disease management.