Pompe Disease Market Report
Published Date: 31 January 2026 | Report Code: pompe-disease
Pompe Disease Market Size, Share, Industry Trends and Forecast to 2033
This report provides a comprehensive analysis of the Pompe Disease market, detailing market size, growth forecasts, industry dynamics, and technological advancements. Insights are projected for the period 2023 to 2033, offering valuable data for stakeholders and decision-makers.
| Metric | Value |
|---|---|
| Study Period | 2023 - 2033 |
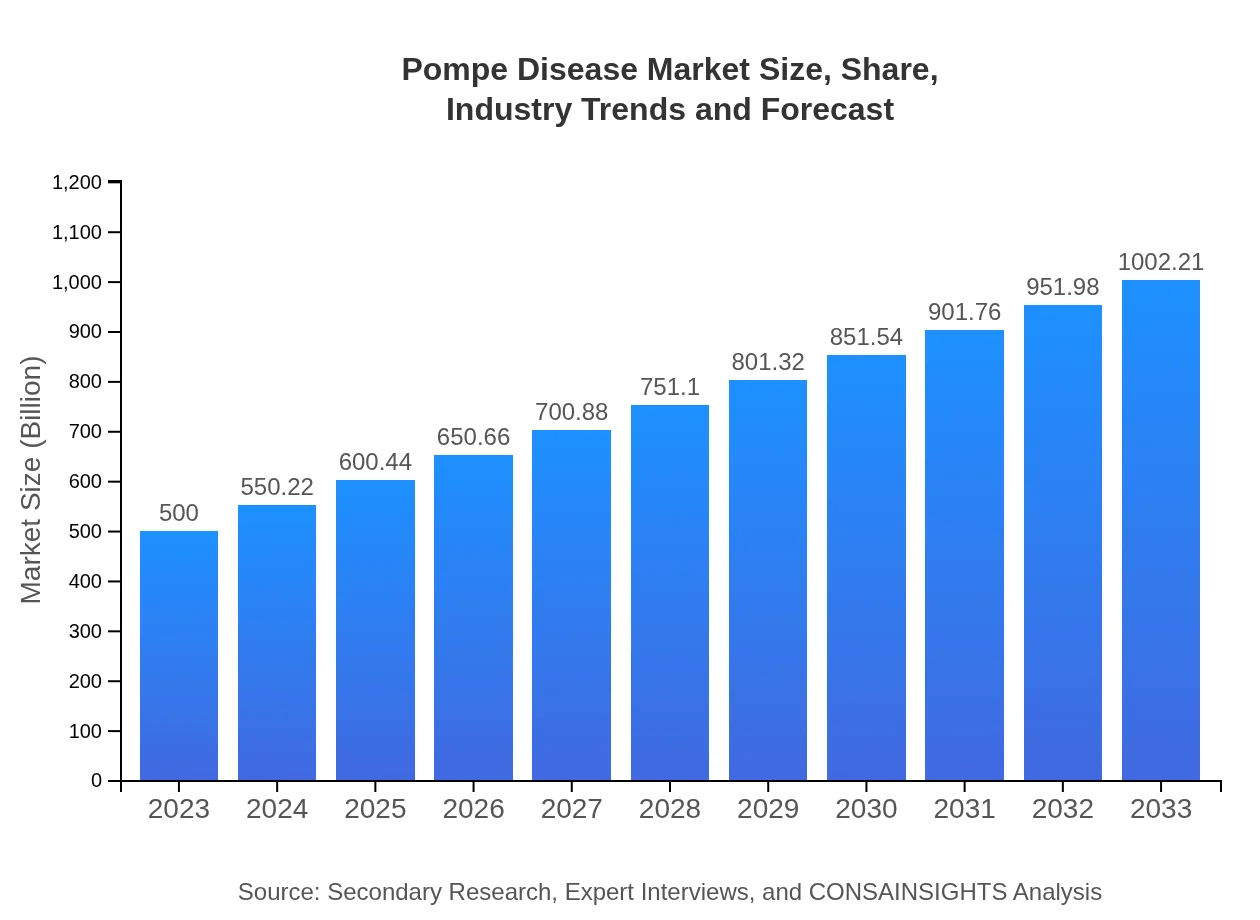
| 2023 Market Size | $500.00 Million |
| CAGR (2023-2033) | 7% |
| 2033 Market Size | $1002.21 Million |
| Top Companies | Sanofi, Amicus Therapeutics, Pfizer , Sobi |
| Last Modified Date | 31 January 2026 |
Pompe Disease Market Overview
Customize Pompe Disease Market Report market research report
- ✔ Get in-depth analysis of Pompe Disease market size, growth, and forecasts.
- ✔ Understand Pompe Disease's regional dynamics and industry-specific trends.
- ✔ Identify potential applications, end-user demand, and growth segments in Pompe Disease
What is the Market Size & CAGR of Pompe Disease market in 2023 and 2033?
Pompe Disease Industry Analysis
Pompe Disease Market Segmentation and Scope
Tell us your focus area and get a customized research report.
Pompe Disease Market Analysis Report by Region
Europe Pompe Disease Market Report:
In Europe, the market size is anticipated to escalate from $121.45 million in 2023 to $243.44 million by 2033. Various funding initiatives from governments to support rare disease treatment research will play a crucial role in market expansion. The region is also witnessing an increase in collaboration between research institutions and biopharmaceutical companies.Asia Pacific Pompe Disease Market Report:
The Asia Pacific region is expected to see significant growth in the Pompe Disease market, with a size of approximately $107.80 million in 2023, projected to reach $216.08 million by 2033. Increased healthcare expenditure, along with rising awareness about Pompe disease among healthcare providers and patients, are contributing to this growth. Emerging economies in this region are also focusing on improving their healthcare infrastructure to address rare diseases.North America Pompe Disease Market Report:
North America holds a significant share of the Pompe Disease market, with current estimates around $185.90 million in 2023, likely doubling to $372.62 million by 2033. The U.S. leads in clinical advancements and has the highest treatment penetration rates. The presence of major global players and favorable regulatory pathways for drug approvals support this growth.South America Pompe Disease Market Report:
In South America, the market for Pompe Disease treatment is estimated to grow from $42.40 million in 2023 to $84.99 million by 2033. The market's growth is hindered by limited access to advanced therapies; however, improvements in healthcare policies and increased funding for rare disease treatments are expected to enhance future prospects in the region.Middle East & Africa Pompe Disease Market Report:
The Middle East and Africa market for Pompe Disease is growing steadily, with size expected to rise from $42.45 million in 2023 to $85.09 million by 2033. The demand for treatment in this region is being driven by higher prevalence rates and ongoing health initiatives aimed at enhancing genetic disease awareness and management.Tell us your focus area and get a customized research report.
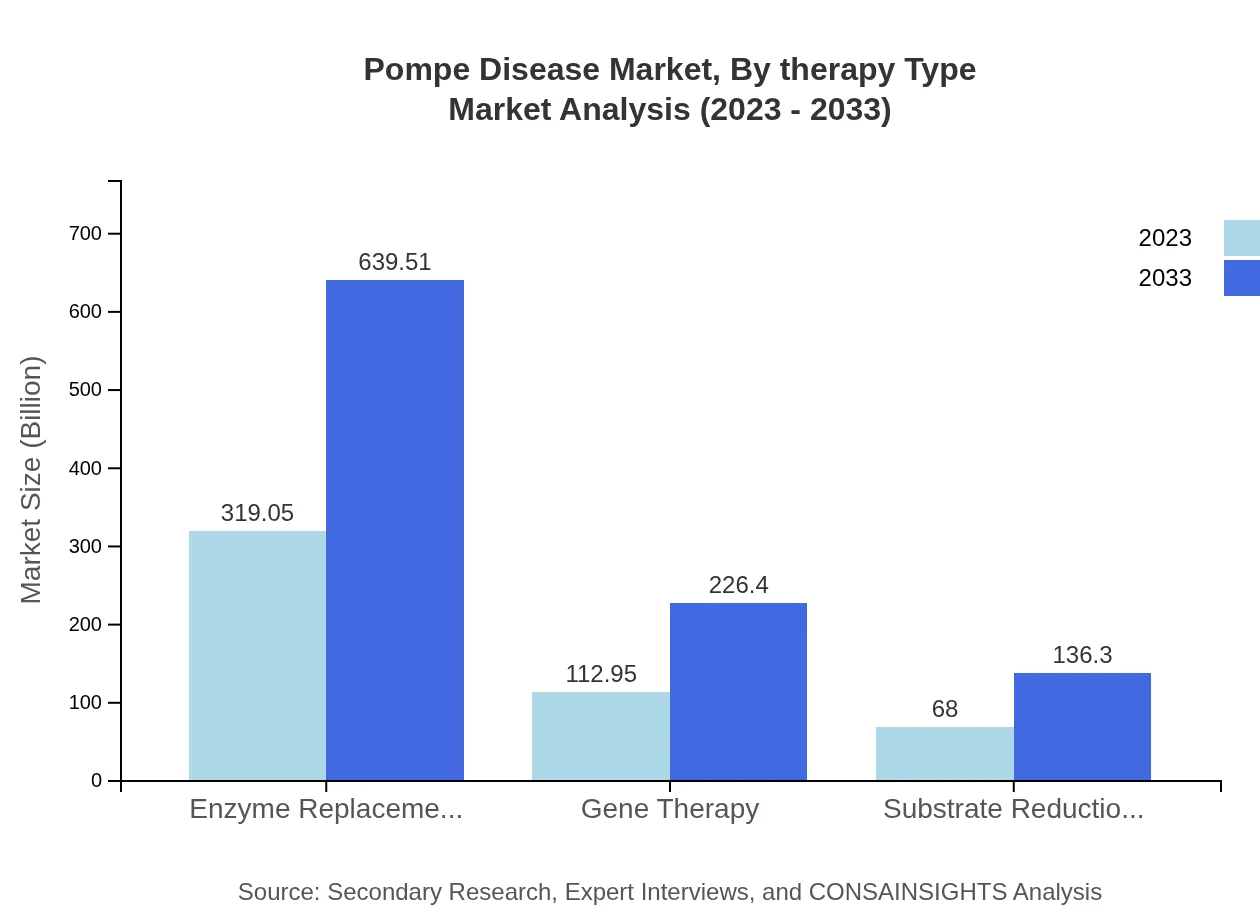
Pompe Disease Market Analysis By Therapy Type
For the Pompe Disease treatment sector, Enzyme Replacement Therapy remains the dominant segment, valued at $319.05 million in 2023 and projected to equal $639.51 million by 2033. Gene Therapy follows, currently at $112.95 million and projecting to $226.40 million, while Substrate Reduction Therapy is expected to grow from $68 million to $136.30 million. This landscape illustrates a significant shift towards innovative therapies that promise improved patient outcomes.
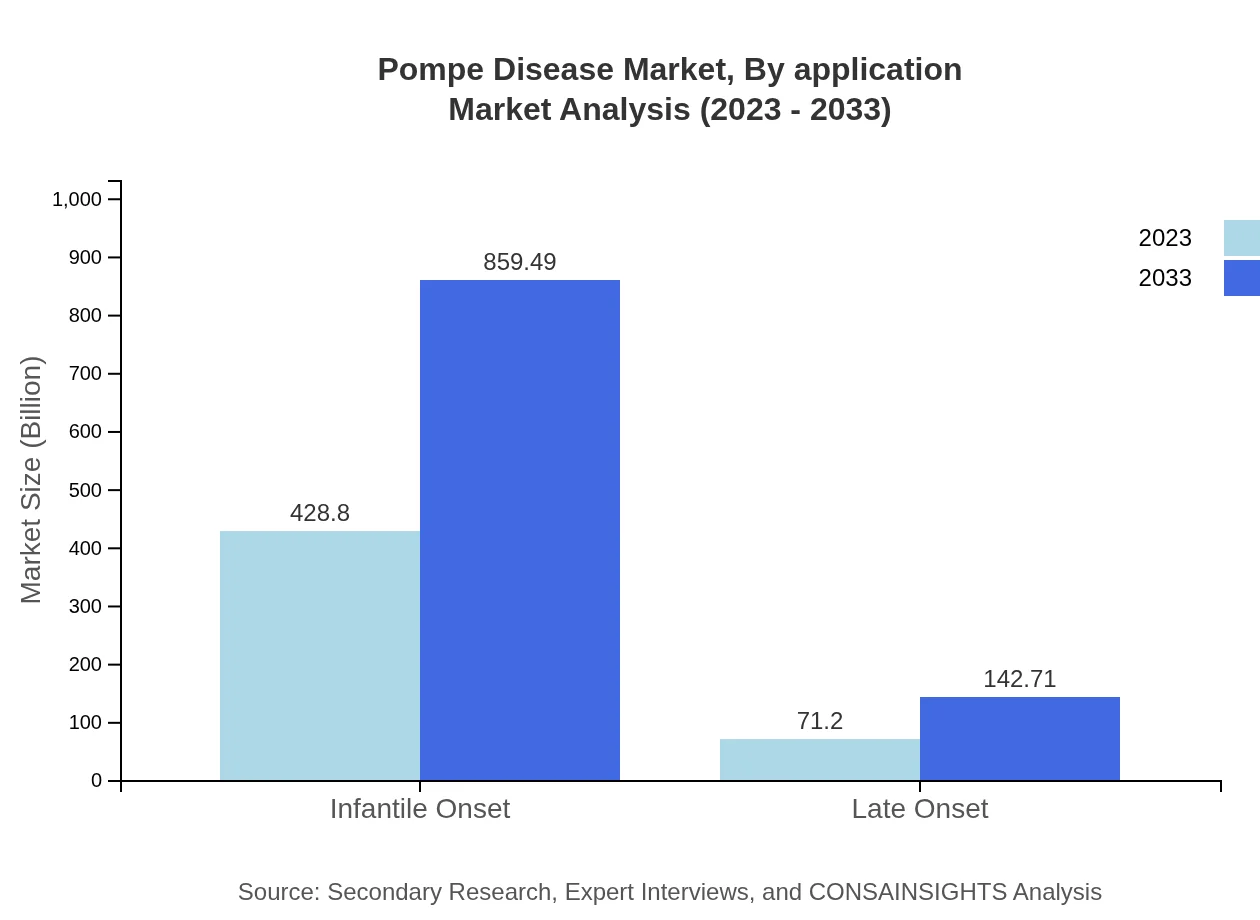
Pompe Disease Market Analysis By Application
The market is largely segmented into Infantile Onset and Late Onset applications, with Infantile Onset treatments accounting for $428.80 million in 2023 and expected to reach $859.49 million by 2033, reflecting greater urgency in treatment needs. Late Onset applications will contribute around $71.20 million at the start, climbing to $142.71 million, indicating growing recognition of the need for comprehensive care across different patient demographics.
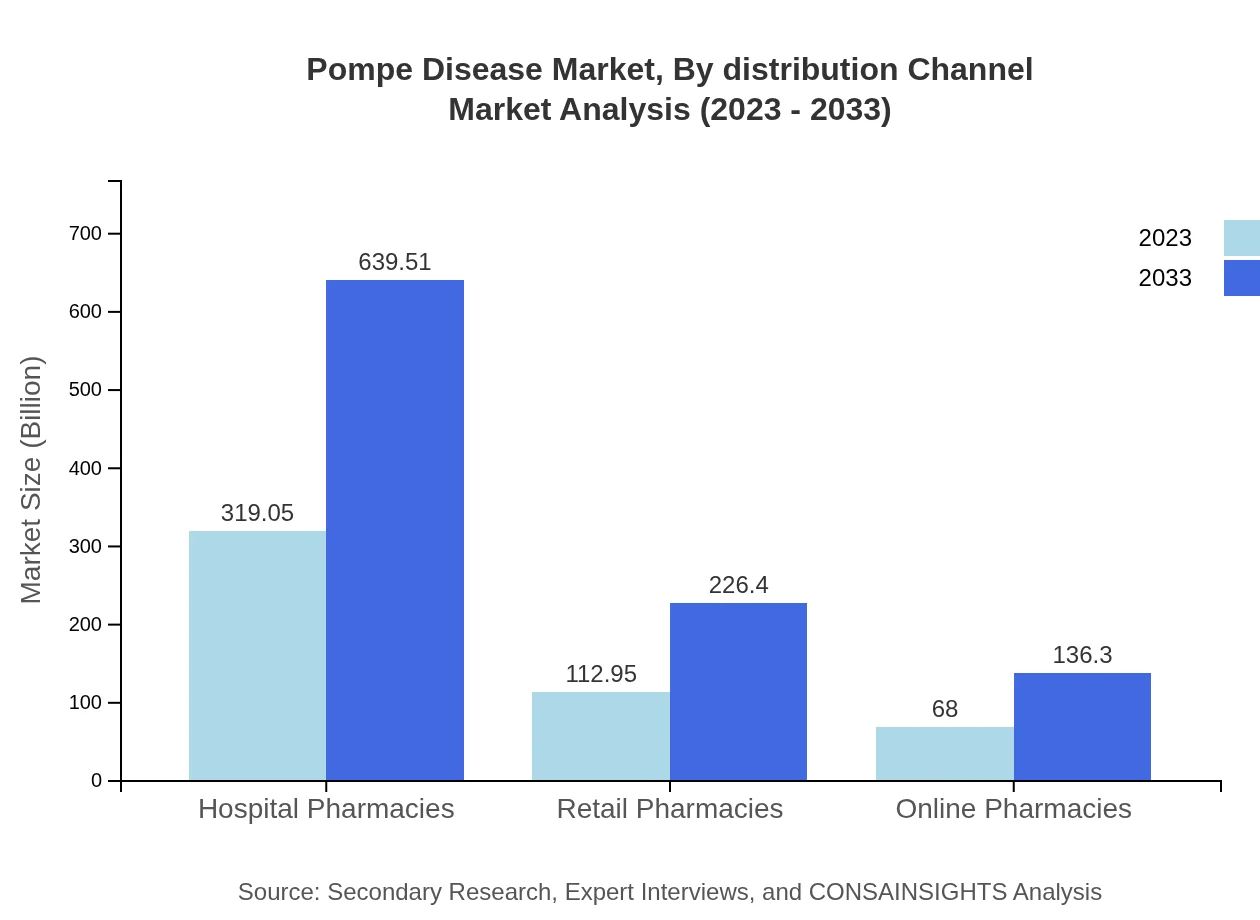
Pompe Disease Market Analysis By Distribution Channel
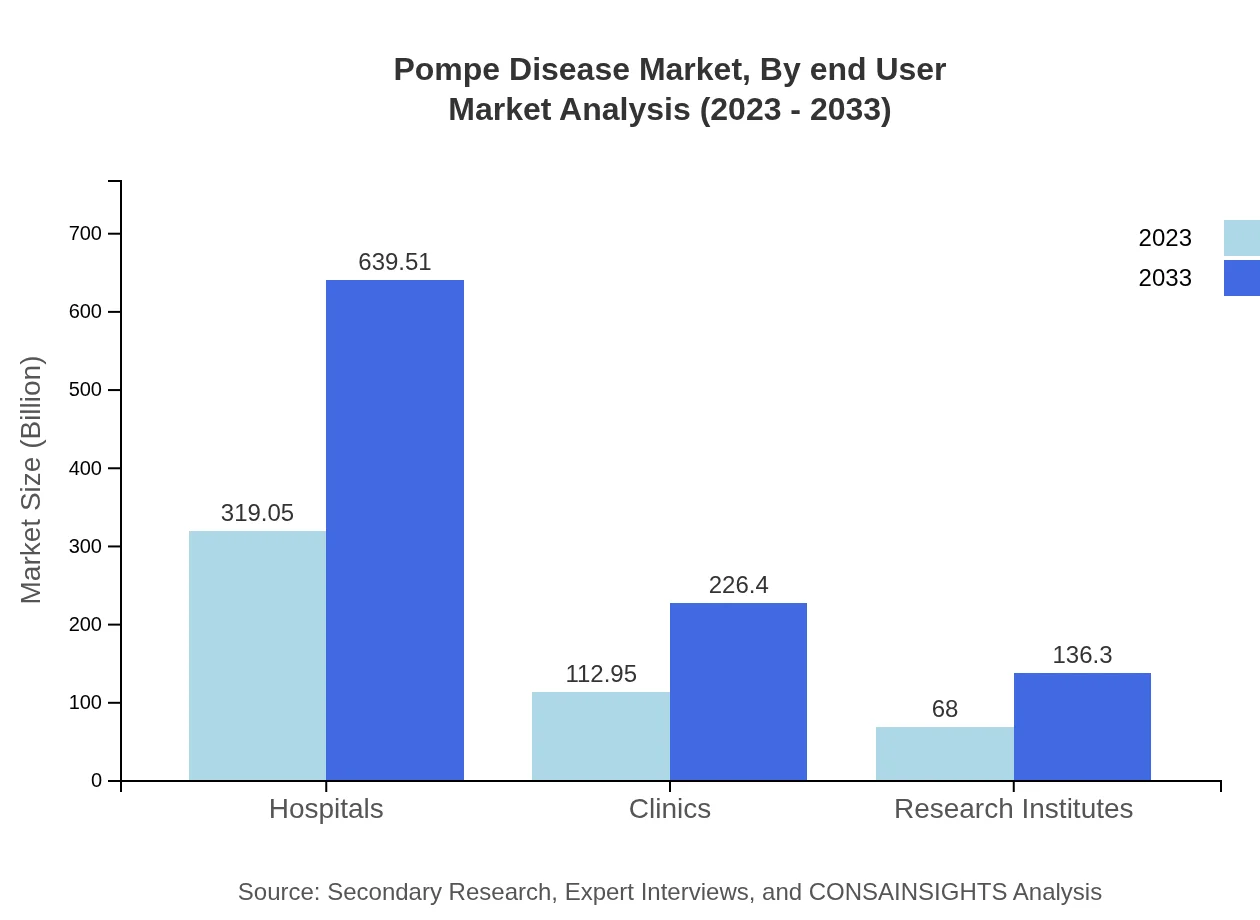
The distribution channels for Pompe Disease therapies include Hospitals, Clinics, Research Institutes, and various pharmacy types. Hospital-based distribution holds the largest market share, valued at $319.05 million in 2023, forecasted to double by 2033. Clinics contribute significantly at $112.95 million initially, with a similar growth trajectory, representing heightened accessibility of treatments to patients.
Pompe Disease Market Analysis By End User
Hospitals and Research Institutes are the primary end users due to their comprehensive treatment facilities. In 2023, Hospitals’ share will be 63.81%, while Research Institutes will account for 13.6% of the market. As the healthcare landscape continues to evolve, the emphasis on research and clinical development will enhance these sectors' significances in managing Pompe Disease.
Pompe Disease Market Trends and Future Forecast
Tell us your focus area and get a customized research report.
Global Market Leaders and Top Companies in Pompe Disease Industry
Sanofi:
A global biopharmaceutical company that offers the leading enzyme replacement therapy for Pompe disease, actively investing in innovative therapies and patient education programs.Amicus Therapeutics:
Focused on developing and delivering gene therapies for genetic diseases, Amicus is pioneering unique treatment approaches for Pompe disease and is engaged in clinical trials.Pfizer :
A major player in the pharmaceutical sector, Pfizer is involved in researching new treatment modalities and collaborating on studies to enhance Pompe disease care.Sobi:
Swedish Orphan Biovitrum has a strong commitment to rare diseases, providing essential therapies and patient support for individuals affected by Pompe disease.We're grateful to work with incredible clients.









FAQs
What is the market size of Pompe disease?
The Pompe disease market is valued at approximately $500 million in 2023, with an expected CAGR of 7% through 2033, indicating robust growth and increasing demand for treatment solutions.
What are the key market players or companies in this Pompe disease industry?
Key players in the Pompe disease market include large pharmaceutical companies focusing on enzyme replacement therapies and gene therapies, which are critical in managing the condition effectively.
What are the primary factors driving the growth in the Pompe disease industry?
Key growth factors include rising awareness about Pompe disease, advancements in genetic research, and the development of innovative therapies contributing to improved patient outcomes worldwide.
Which region is the fastest Growing in the Pompe disease?
North America is the fastest-growing region for Pompe disease, projected to grow from $185.90 million in 2023 to $372.62 million by 2033, reflecting a significant demand for treatment.
Does ConsaInsights provide customized market report data for the Pompe disease industry?
Yes, ConsaInsights offers customized market report data tailored specifically to the unique needs and interests of stakeholders in the Pompe disease industry, ensuring actionable insights.
What deliverables can I expect from this Pompe disease market research project?
Expect comprehensive market reports including trends, forecasts, regional data, competitive analysis, and actionable recommendations tailored for the Pompe disease sector.
What are the market trends of Pompe disease?
Current trends in the Pompe disease market include increasing focus on gene therapy developments, expansion of enzyme replacement therapies, and heightened regulatory support for innovative treatments.