Postmenopausal Vaginal Atrophy Drugs Market Report
Published Date: 31 January 2026 | Report Code: postmenopausal-vaginal-atrophy-drugs
Postmenopausal Vaginal Atrophy Drugs Market Size, Share, Industry Trends and Forecast to 2033
This report provides an in-depth analysis of the Postmenopausal Vaginal Atrophy Drugs market from 2023 to 2033, covering market size, growth trends, segmentation, and regional insights, along with key players and future forecasts.
| Metric | Value |
|---|---|
| Study Period | 2023 - 2033 |
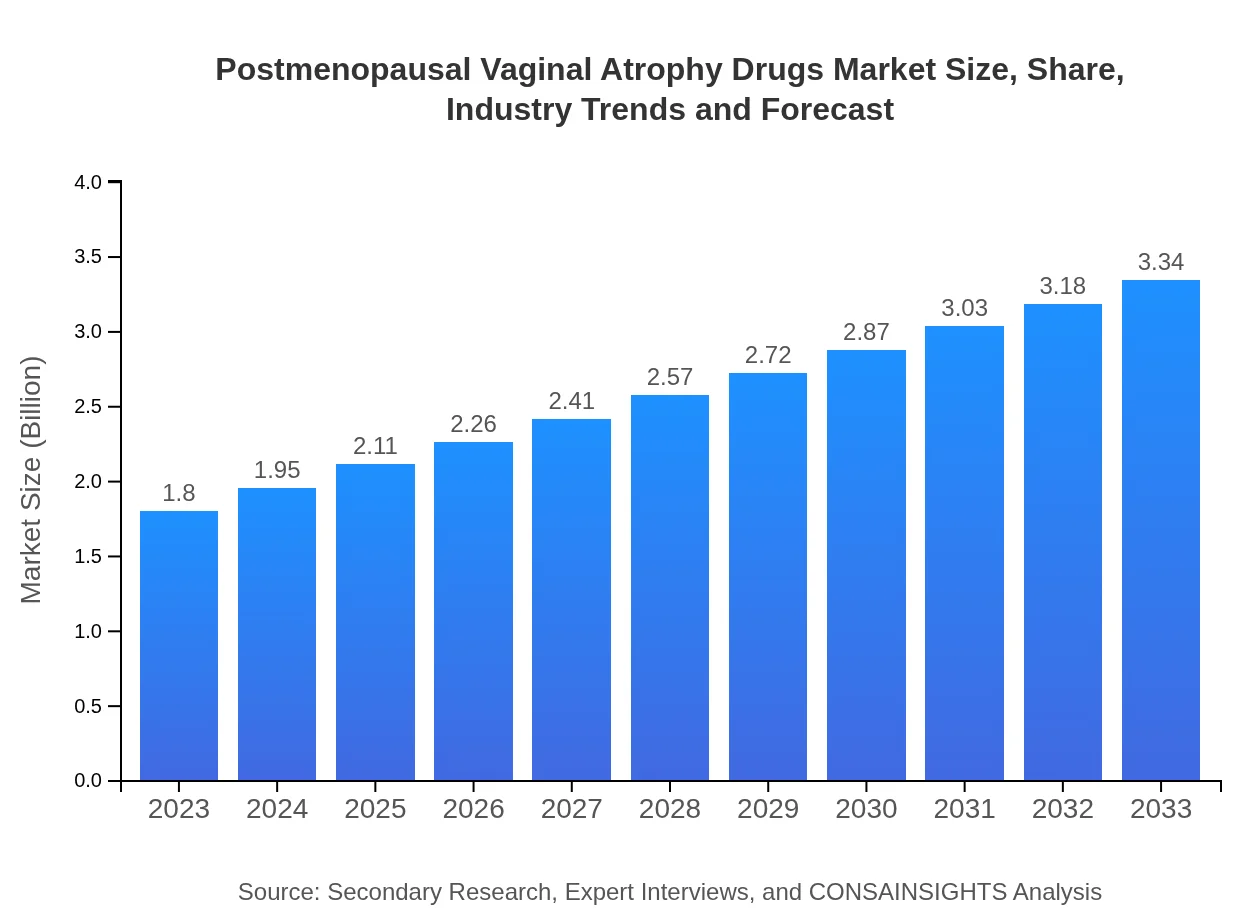
| 2023 Market Size | $1.80 Billion |
| CAGR (2023-2033) | 6.2% |
| 2033 Market Size | $3.34 Billion |
| Top Companies | Pfizer Inc., Bristol-Myers Squibb, Amgen Inc., Bayer AG, Hologic, Inc. |
| Last Modified Date | 31 January 2026 |
Postmenopausal Vaginal Atrophy Drugs Market Overview
Customize Postmenopausal Vaginal Atrophy Drugs Market Report market research report
- ✔ Get in-depth analysis of Postmenopausal Vaginal Atrophy Drugs market size, growth, and forecasts.
- ✔ Understand Postmenopausal Vaginal Atrophy Drugs's regional dynamics and industry-specific trends.
- ✔ Identify potential applications, end-user demand, and growth segments in Postmenopausal Vaginal Atrophy Drugs
What is the Market Size & CAGR of Postmenopausal Vaginal Atrophy Drugs market in 2023?
Postmenopausal Vaginal Atrophy Drugs Industry Analysis
Postmenopausal Vaginal Atrophy Drugs Market Segmentation and Scope
Tell us your focus area and get a customized research report.
Postmenopausal Vaginal Atrophy Drugs Market Analysis Report by Region
Europe Postmenopausal Vaginal Atrophy Drugs Market Report:
The European market was valued at $0.59 billion in 2023, with forecasts indicating a rise to $1.09 billion by 2033. Increased healthcare expenditure and product availability aid this growth.Asia Pacific Postmenopausal Vaginal Atrophy Drugs Market Report:
In 2023, the Asia Pacific market was valued at $0.33 billion, projected to reach $0.61 billion by 2033. The increasing population of aging women and awareness of treatment options are significant growth drivers in this region.North America Postmenopausal Vaginal Atrophy Drugs Market Report:
North America holds a market size of $0.65 billion in 2023 and is projected to expand to $1.21 billion by 2033. The high prevalence of PVA due to an aging population and comprehensive healthcare systems support robust market growth.South America Postmenopausal Vaginal Atrophy Drugs Market Report:
The South American market was valued at $0.17 billion in 2023 and is expected to hit $0.32 billion by 2033. The focus on healthcare improvements and access to treatment options is fostering growth.Middle East & Africa Postmenopausal Vaginal Atrophy Drugs Market Report:
The Middle East and Africa market is smaller, valued at $0.06 billion in 2023, expected to grow to $0.11 billion by 2033, driven by gradual improvements in healthcare infrastructure.Tell us your focus area and get a customized research report.
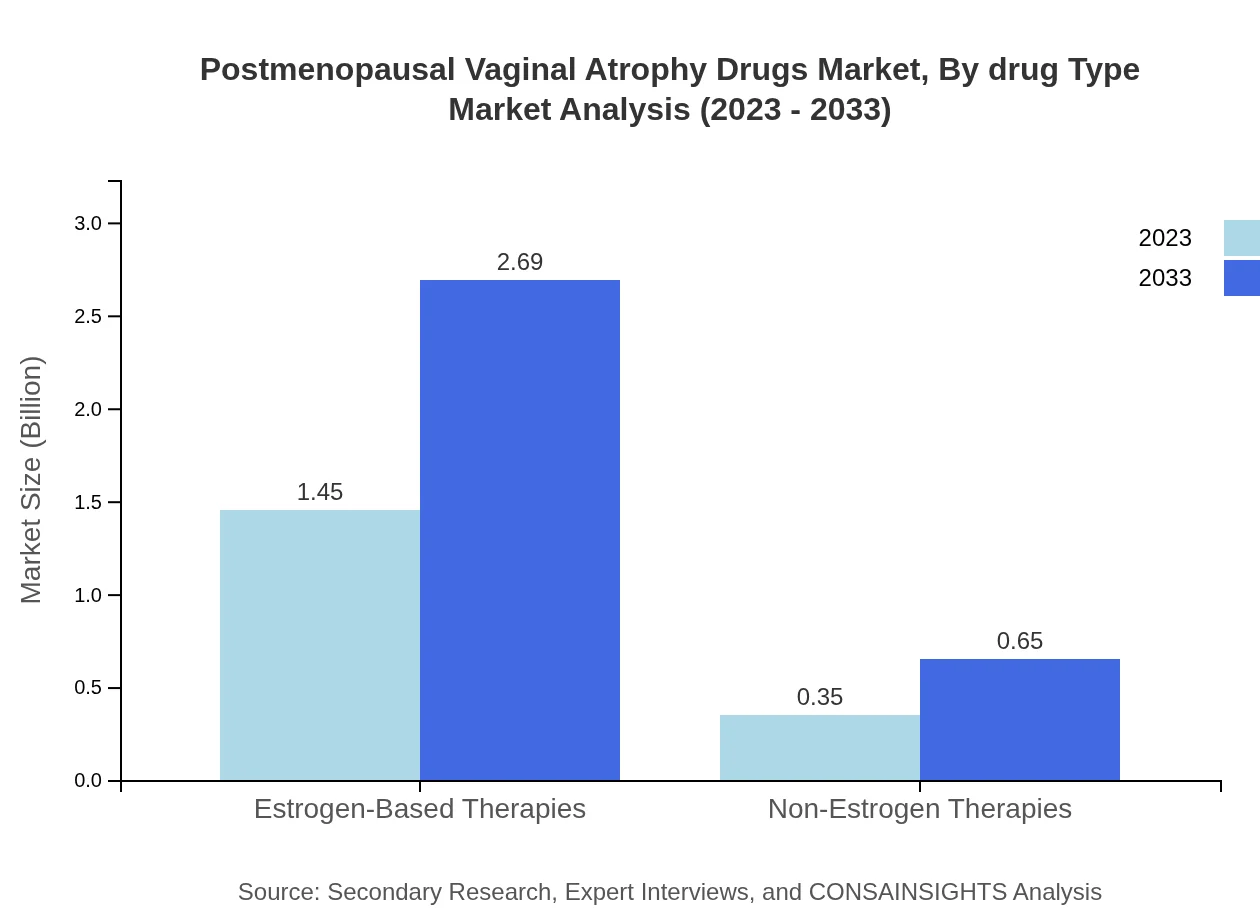
Postmenopausal Vaginal Atrophy Drugs Market Analysis By Drug Type
Estrogen-based therapies dominate the market, accounting for $1.45 billion in 2023 and projected to reach $2.69 billion by 2033, representing an 80.6% market share. Non-estrogen therapies comprise $0.35 billion in 2023 with expectations to grow to $0.65 billion, indicating a niche but expanding segment.
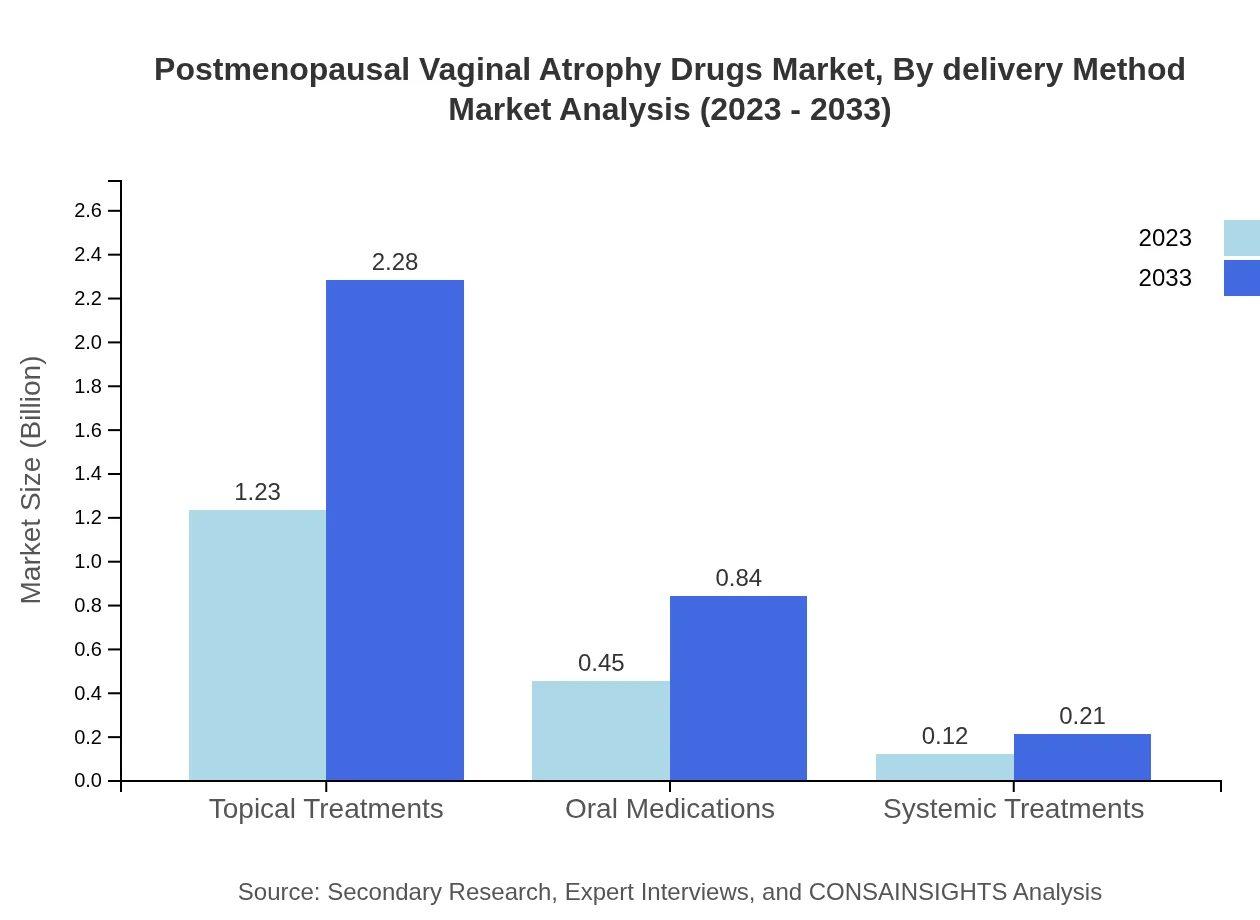
Postmenopausal Vaginal Atrophy Drugs Market Analysis By Delivery Method
Topical treatments, with a market size of $1.23 billion in 2023, hold a significant share at 68.5%, expected to reach $2.28 billion in 2033. Oral medications follow, at $0.45 billion in 2023, with a forecasted increase to $0.84 billion.
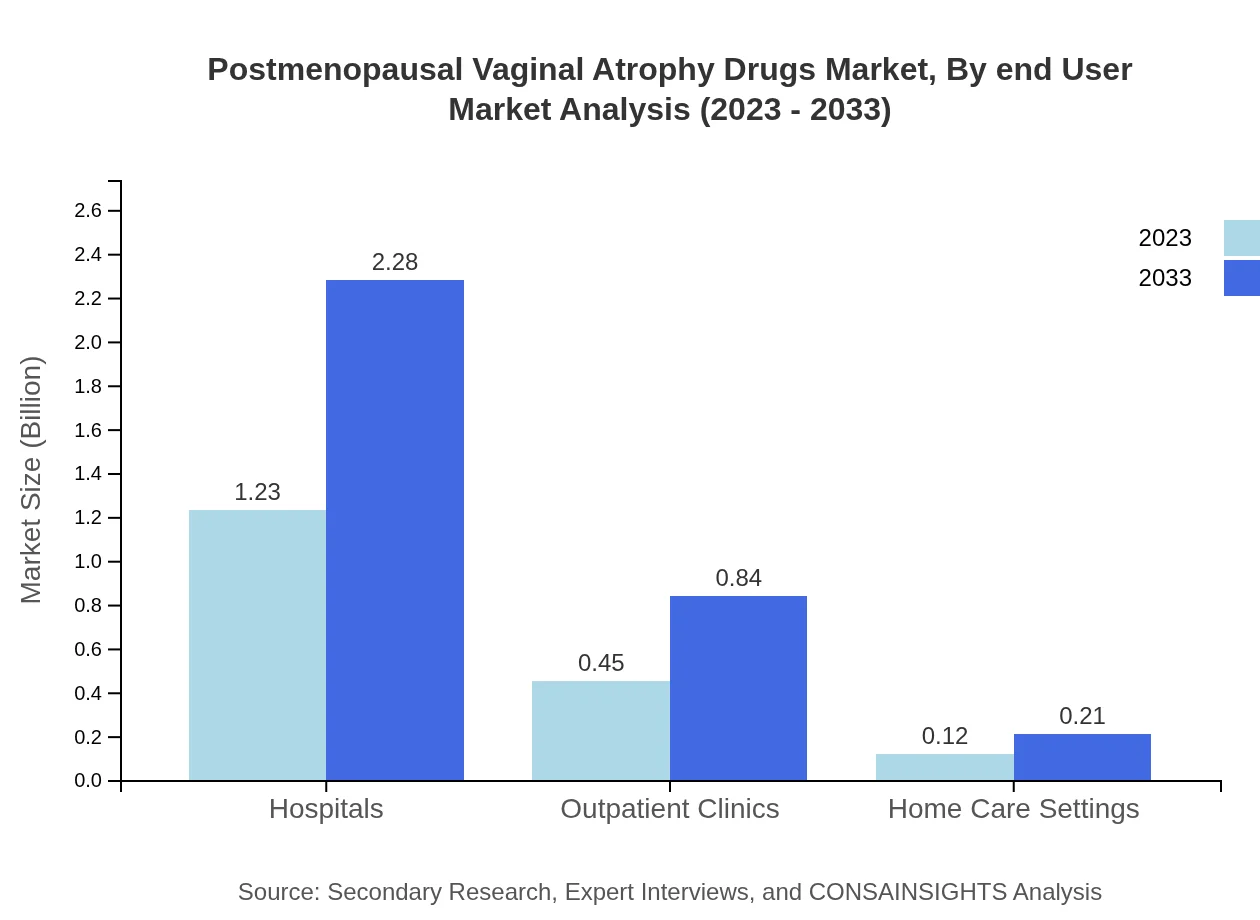
Postmenopausal Vaginal Atrophy Drugs Market Analysis By End User
Hospitals lead the market at $1.23 billion in 2023, holding a 68.5% market share. Outpatient clinics, generating $0.45 billion in 2023, are projected to grow to $0.84 billion. Home care settings represent a smaller segment, valued at $0.12 billion in 2023.
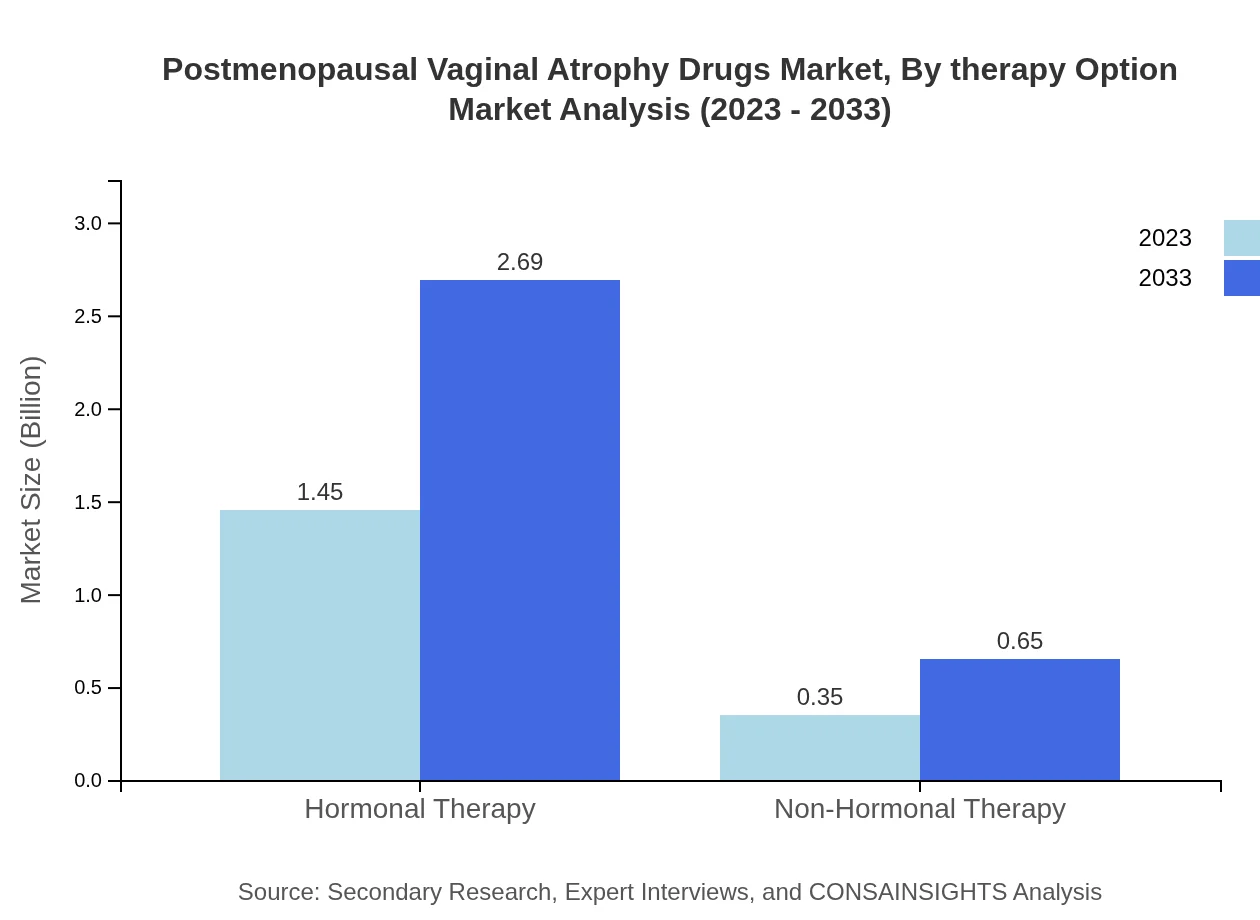
Postmenopausal Vaginal Atrophy Drugs Market Analysis By Therapy Option
Hormonal therapy is projected to account for $1.45 billion in 2023 with a significant market share of 80.6%, growing to $2.69 billion by 2033. Conversely, non-hormonal therapy, valued at $0.35 billion, is expected to expand to $0.65 billion.
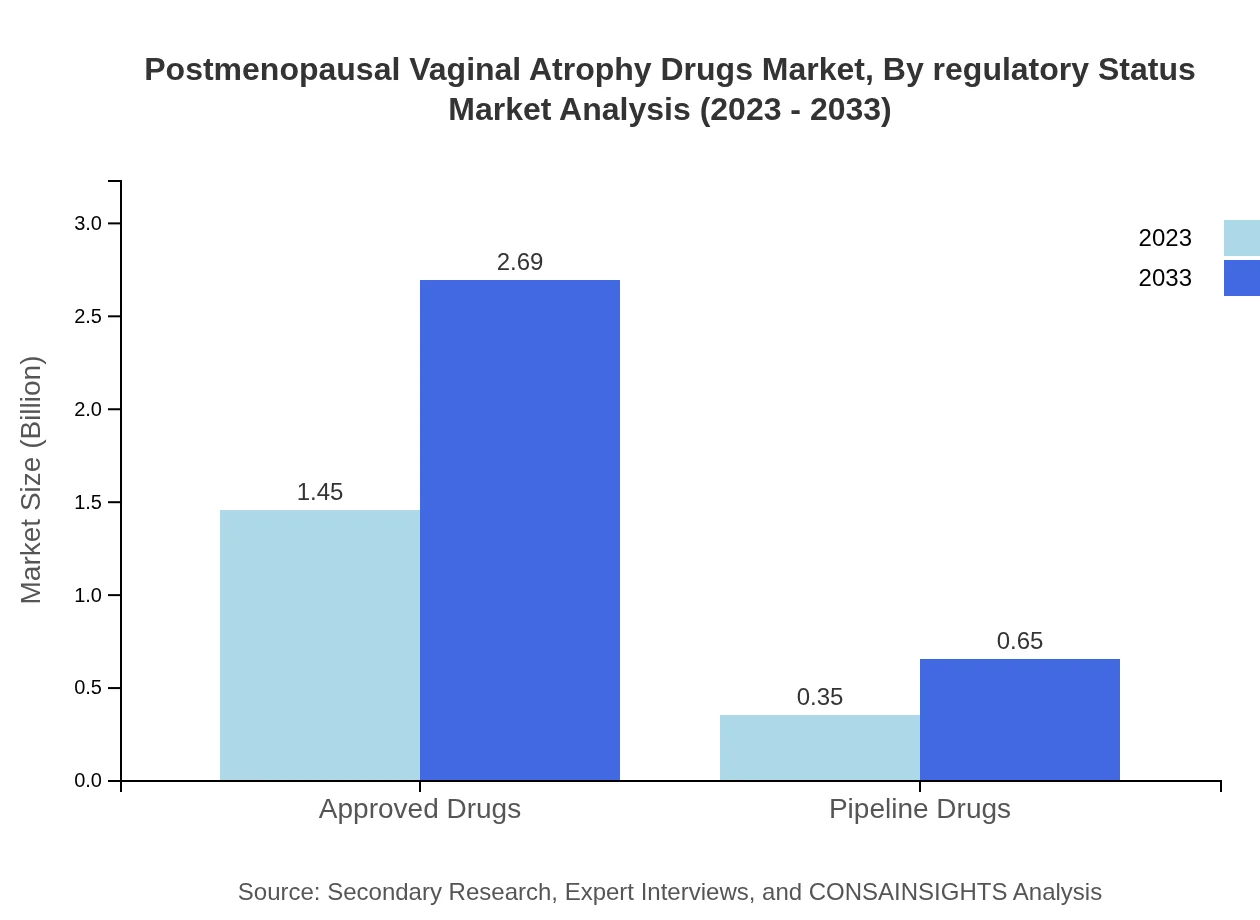
Postmenopausal Vaginal Atrophy Drugs Market Analysis By Regulatory Status
The segmentation into approved drugs versus pipeline drugs shows a continuous demand for established treatments, with approved drugs accounting for $1.45 billion in 2023 and projected growth to $2.69 billion, maintaining an 80.6% share.
Postmenopausal Vaginal Atrophy Drugs Market Trends and Future Forecast
Tell us your focus area and get a customized research report.
Global Market Leaders and Top Companies in Postmenopausal Vaginal Atrophy Drugs Industry
Pfizer Inc.:
A pivotal player in the PVA drugs market, Pfizer is known for drugs that effectively manage estrogen deficiency-related symptoms, ensuring widespread availability and efficacy.Bristol-Myers Squibb:
Bristol-Myers Squibb specializes in innovative treatments for women's health, developing non-hormonal options for vaginal atrophy, contributing significantly to expanding market choices.Amgen Inc.:
Known for its focus on biological products, Amgen develops therapies aimed at reducing the symptoms of vaginal atrophy, adding diversity to treatment protocols.Bayer AG:
Bayer is instrumental in providing a range of hormonal therapies tailored to treat postmenopausal conditions, impacting millions of women's health globally.Hologic, Inc.:
Focused on women’s health, Hologic offers comprehensive solutions for vaginal dryness and atrophy, expanding treatment frameworks through innovative devices and drugs.We're grateful to work with incredible clients.









FAQs
What is the market size of postmenopausal Vaginal Atrophy Drugs?
The postmenopausal vaginal atrophy drugs market is valued at approximately $1.8 billion in 2023 and is projected to grow at a CAGR of 6.2% through 2033, indicating robust growth in demand for treatment options.
What are the key market players or companies in this postmenopausal Vaginal Atrophy Drugs industry?
Key players in the postmenopausal vaginal atrophy drugs market include pharmaceutical companies specializing in women's health, though specific names are not listed, these companies drive innovation and development of therapies like estrogen-based products and non-hormonal alternatives.
What are the primary factors driving the growth in the postmenopausal Vaginal Atrophy Drugs industry?
Factors driving growth include increasing awareness of menopausal health, rising aging female demographics, and advancements in drug formulations, alongside a growing acceptance of treatment options among postmenopausal women.
Which region is the fastest Growing in the postmenopausal Vaginal Atrophy Drugs?
North America is currently the fastest-growing region, with a projected market size increase from $0.65 billion in 2023 to $1.21 billion by 2033, reflecting substantial growth in addressing women's reproductive health.
Does ConsaInsights provide customized market report data for the postmenopausal Vaginal Atrophy Drugs industry?
Yes, ConsaInsights offers customized market report data tailored to specific needs within the postmenopausal vaginal atrophy drugs industry, helping clients gain insights into niche segments and growth opportunities.
What deliverables can I expect from this postmenopausal Vaginal Atrophy Drugs market research project?
Expect deliverables including detailed market reports featuring size, growth forecasts, competitive landscape analysis, trends, and insights segmented by region and market dynamics within the postmenopausal vaginal atrophy space.
What are the market trends of postmenopausal Vaginal Atrophy Drugs?
Market trends include increasing preference for topical treatments, growing demand for non-hormonal therapies, and a shift towards personalized medicine, as well as greater focus on patient education and holistic health approaches.