Postpartum Hemorrhage Pph Devices Market Report
Published Date: 31 January 2026 | Report Code: postpartum-hemorrhage-pph-devices
Postpartum Hemorrhage Pph Devices Market Size, Share, Industry Trends and Forecast to 2033
This report provides a comprehensive analysis of the Postpartum Hemorrhage (PPH) Devices market from 2023 to 2033, including market conditions, size, CAGR forecasts, regional insights, technological trends, and key market players.
| Metric | Value |
|---|---|
| Study Period | 2023 - 2033 |
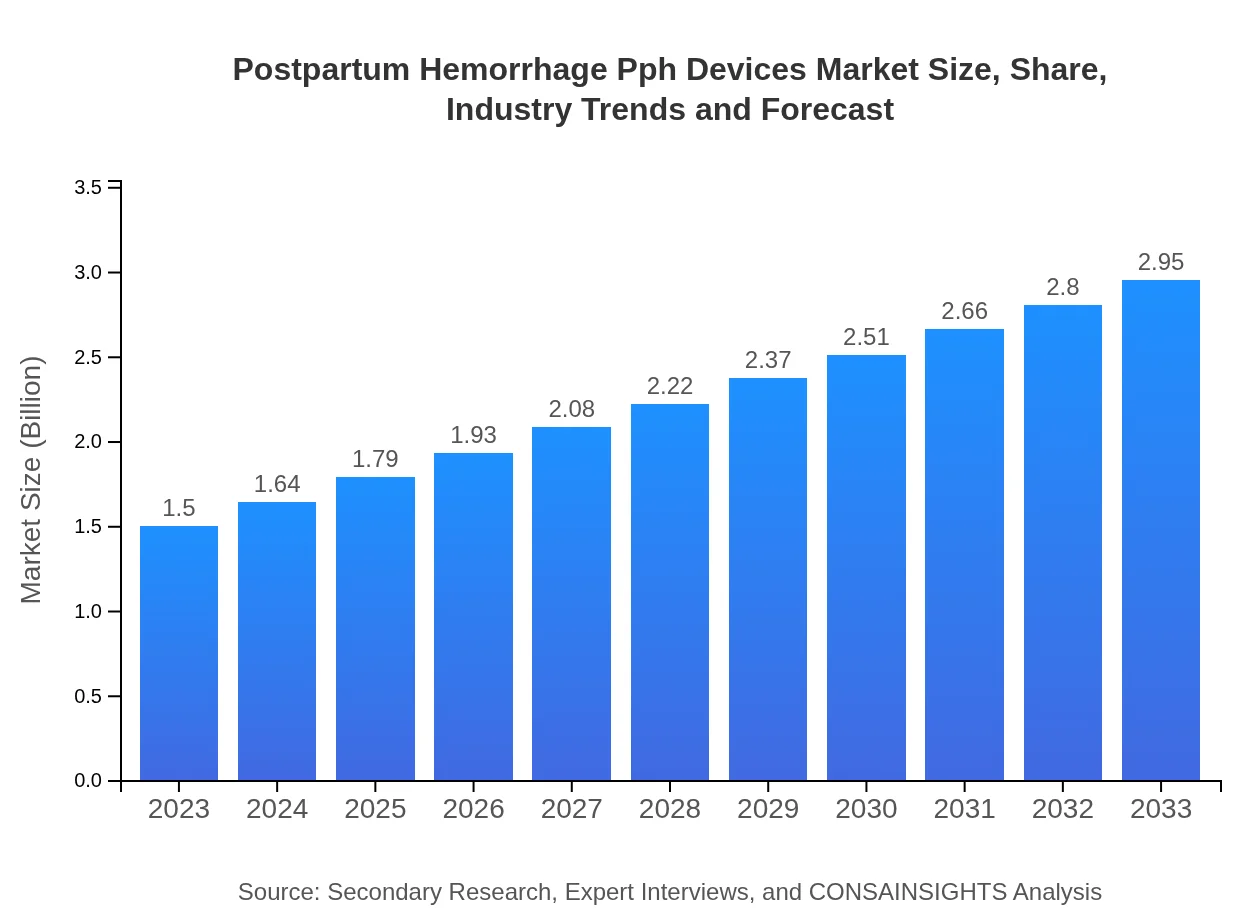
| 2023 Market Size | $1.50 Billion |
| CAGR (2023-2033) | 6.8% |
| 2033 Market Size | $2.95 Billion |
| Top Companies | Baxter International, Medtronic , Johnson & Johnson, Stryker Corporation |
| Last Modified Date | 31 January 2026 |
Postpartum Hemorrhage Pph Devices Market Overview
Customize Postpartum Hemorrhage Pph Devices Market Report market research report
- ✔ Get in-depth analysis of Postpartum Hemorrhage Pph Devices market size, growth, and forecasts.
- ✔ Understand Postpartum Hemorrhage Pph Devices's regional dynamics and industry-specific trends.
- ✔ Identify potential applications, end-user demand, and growth segments in Postpartum Hemorrhage Pph Devices
What is the Market Size & CAGR of Postpartum Hemorrhage Pph Devices market in 2023?
Postpartum Hemorrhage Pph Devices Industry Analysis
Postpartum Hemorrhage Pph Devices Market Segmentation and Scope
Tell us your focus area and get a customized research report.
Postpartum Hemorrhage Pph Devices Market Analysis Report by Region
Europe Postpartum Hemorrhage Pph Devices Market Report:
In Europe, the market is expected to rise from $0.45 billion in 2023 to $0.88 billion by 2033, strengthened by supportive government policies, awareness campaigns, and a rising number of childbirths, combined with effective maternal care strategies.Asia Pacific Postpartum Hemorrhage Pph Devices Market Report:
In the Asia Pacific region, the PPH Devices market is projected to grow from $0.28 billion in 2023 to $0.56 billion by 2033, marking a substantial increase due to rising healthcare expenditures and growing awareness about maternal health.North America Postpartum Hemorrhage Pph Devices Market Report:
North America has a significant market for PPH Devices, anticipated to grow from $0.57 billion in 2023 to $1.11 billion by 2033, supported by advanced healthcare infrastructure, high adoption rates of innovative technologies, and robust R&D.South America Postpartum Hemorrhage Pph Devices Market Report:
The South American market is set to expand from $0.10 billion in 2023 to $0.19 billion by 2033, driven by healthcare improvements and efforts to reduce maternal mortality, albeit at a slower pace compared to more developed regions.Middle East & Africa Postpartum Hemorrhage Pph Devices Market Report:
The Middle East and Africa are forecasted to see an increase from $0.11 billion in 2023 to $0.21 billion by 2033, as healthcare systems evolve and focus increasingly on maternal health amidst challenging healthcare landscapes.Tell us your focus area and get a customized research report.
Postpartum Hemorrhage Pph Devices Market Analysis By Product
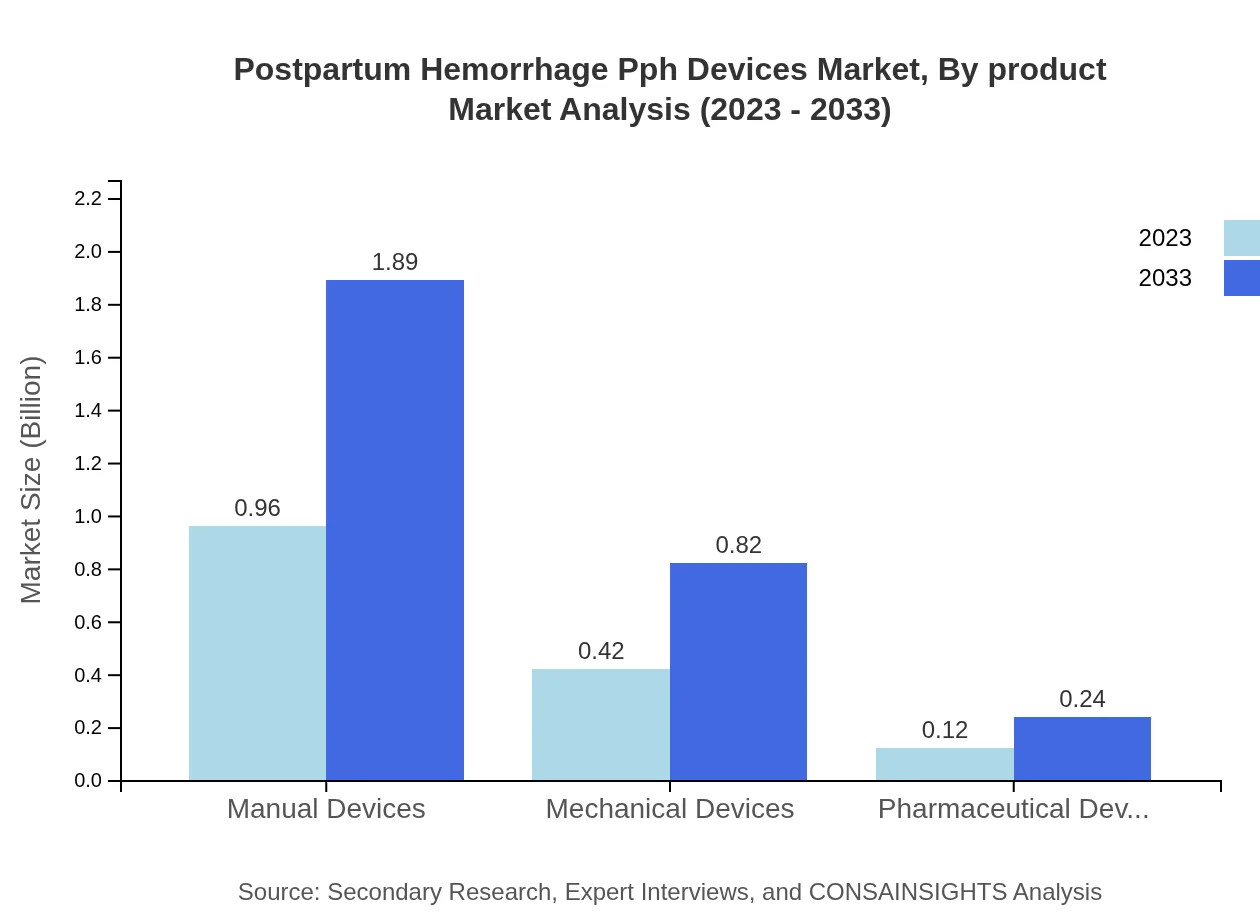
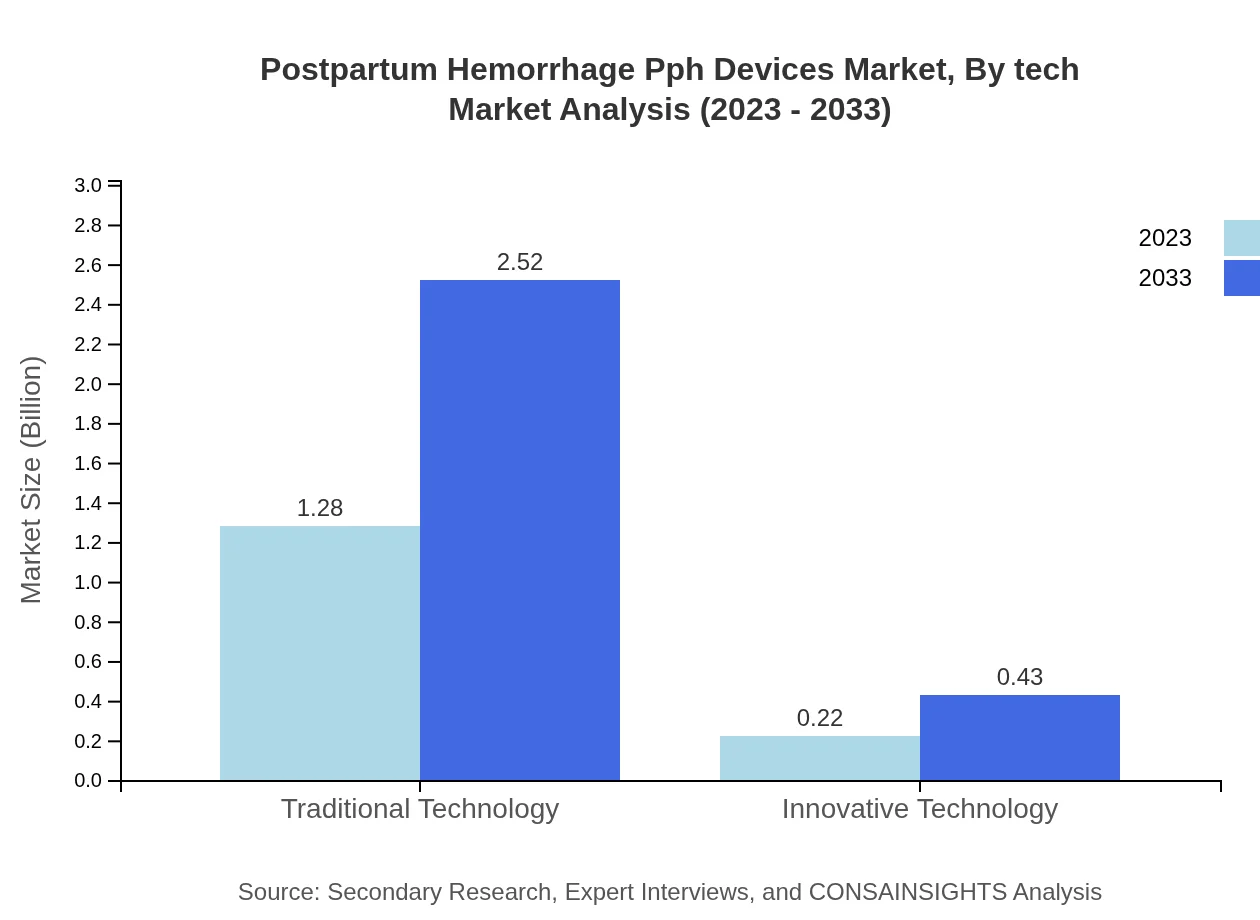
The product type analysis reveals that traditional devices dominate the market with a size of $1.28 billion in 2023 projected to reach $2.52 billion by 2033, holding a significant share of 85.53%. Innovative technologies, while smaller in value at $0.22 billion currently, are gaining a foothold with an estimated future size of $0.43 billion, representing a 14.47% market share. Manual devices represent another strong segment with a market size moving from $0.96 billion to $1.89 billion, and mechanical devices follow closely, projected to grow from $0.42 billion to $0.82 billion, indicating shifting preferences toward advanced solutions.
Postpartum Hemorrhage Pph Devices Market Analysis By Application
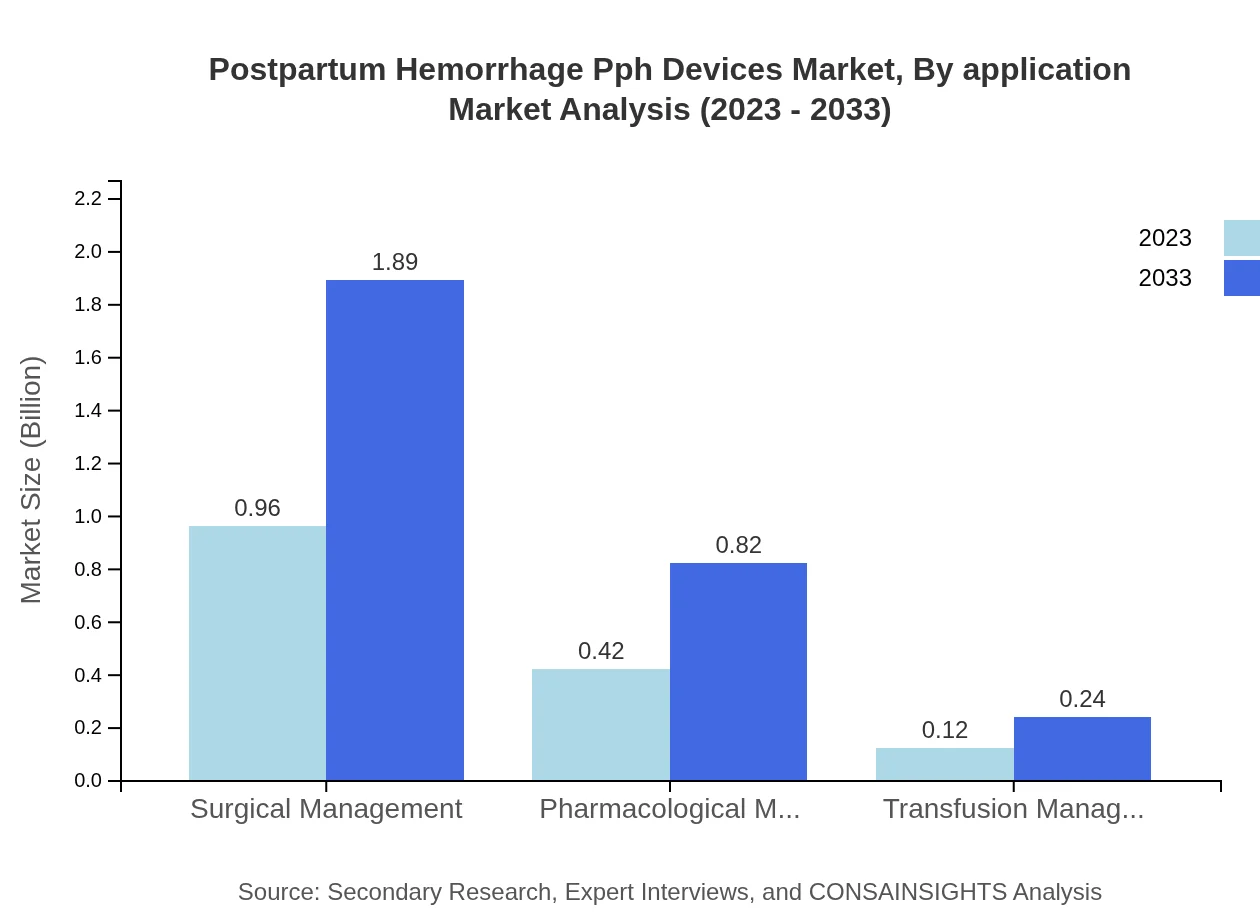
By application, surgical management takes the lead with a market size of $0.96 billion in 2023, expected to double to $1.89 billion by 2033, covering a substantial share of 64.13%. Pharmacological management is set to grow from $0.42 billion to $0.82 billion, marking a share of 27.75%, while transfusion management remains critical but represents a smaller segment with $0.12 billion in 2023, anticipated to reach $0.24 billion by 2033.
Postpartum Hemorrhage Pph Devices Market Analysis By End User
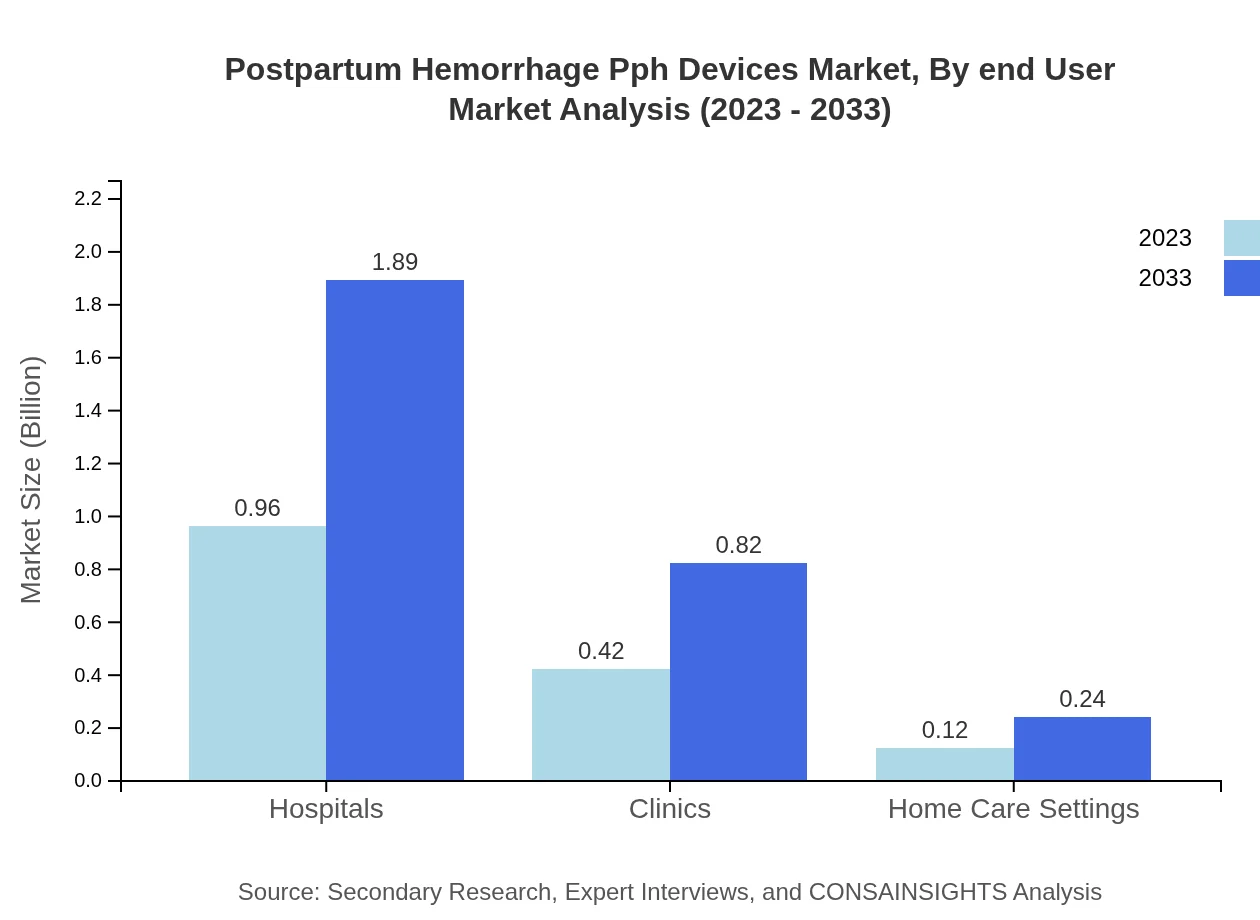
In terms of end-users, hospitals dominate utilization, with a market size of $0.96 billion in 2023 and an expected growth to $1.89 billion. Clinics also maintain a considerable market presence with $0.42 billion, depicting the need for PPH solutions in outpatient settings. Home care settings, while smaller at $0.12 billion, indicate a growing trend of at-home maternal care in developing regions.
Postpartum Hemorrhage Pph Devices Market Analysis By Tech
The technology segmentation highlights traditional methods leading with a size of $1.28 billion, emphasizing their relevance despite the rising interest in innovative approaches which are expected to grow moderately in market size from $0.22 billion to $0.43 billion. The steady performance of mechanical and manual devices reinforces their importance in existing healthcare facilities.
Postpartum Hemorrhage Pph Devices Market Trends and Future Forecast
Tell us your focus area and get a customized research report.
Global Market Leaders and Top Companies in Postpartum Hemorrhage Pph Devices Industry
Baxter International:
A leader in healthcare, Baxter offers innovative PPH solutions and therapies focusing on critical care and pain management.Medtronic :
Medtronic is renowned for its cutting-edge technologies and devices used in surgical management of PPH, contributing significantly to the industry's growth.Johnson & Johnson:
As a major player in the medical device market, Johnson & Johnson's extensive product lineup includes crucial solutions aimed at managing postpartum hemorrhage.Stryker Corporation:
Stryker specializes in surgical devices and technologies that enhance surgical procedures related to PPH management.We're grateful to work with incredible clients.









FAQs
What is the market size of postpartum Hemorrhage Pph Devices?
The Postpartum Hemorrhage (PPH) Devices market is valued at approximately $1.5 billion in 2023, with a significant growth trajectory anticipated, projected to expand at a CAGR of 6.8% through 2033.
What are the key market players or companies in the postpartum Hemorrhage Pph Devices industry?
Key players in the PPH devices market typically include major medical device manufacturers and companies specializing in maternal health technologies. Specific leaders often comprise those dedicated to innovative solutions and various established brands in obstetric care.
What are the primary factors driving the growth in the postpartum Hemorrhage Pph Devices industry?
Growth in the PPH devices market is primarily driven by increasing awareness of maternal health, advances in technology, rise in maternal mortality due to hemorrhage, and growing incidence rates of childbirth complications in various demographics.
Which region is the fastest Growing in the postpartum Hemorrhage Pph Devices?
The North America region is the fastest-growing market for PPH devices, expected to expand from $0.57 billion in 2023 to $1.11 billion by 2033, driven by advanced healthcare infrastructure and increased focus on maternal safety.
Does ConsaInsights provide customized market report data for the postpartum Hemorrhage Pph Devices industry?
Yes, ConsaInsights offers customized market report data for the postpartum hemorrhage devices sector, allowing clients to obtain tailored insights based on specific needs, including geographic focus and product segments.
What deliverables can I expect from this postpartum Hemorrhage Pph Devices market research project?
From this PPH market research project, expect comprehensive reports detailing market size, growth forecasts, competitor analysis, regional insights, segmentation data, and strategic recommendations for market entry or expansion.
What are the market trends of postpartum Hemorrhage Pph Devices?
Current market trends in PPH devices include increased adoption of innovative technologies, a shift towards multi-disciplinary approaches in maternal care, emphasis on preventive measures, and rising investments in healthcare infrastructure.